Primary amyloidosis is a plasma cell dyscrasia with tissue deposition of abnormally folded (β-pleated) immunoglobulin light chains, or rarely, heavy chains.1 The resultant organ dysfunction leads to significant morbidity and mortality. Cardiac disease is the leading cause of death in amyloidosis. Indicators of poor prognosis include hepatomegaly, elevated serum creatinine, significant weight loss, urinary λ light-chain excretion, and elevated β-2 microglobulin. A commonly used hematologic prognostic factor is the difference between the involved and uninvolved free light-chain level (dFLC), with a cutoff of 180 mg/dL.2 The plasma cell burden in marrow biopsies from patients with amyloid light-chain (AL) amyloidosis is highly variable ranging from no overt increase in plasma cells, to frank myeloma.1
Drs. Eli Muchtar and colleagues evaluated the utility of multiparameteric flow cytometry in patients with AL amyloidosis at diagnosis (N=173) and at the end of the first line of treatment (N=82). They evaluated two flow cytometry generated parameters: the percentage of monotypic plasma cells and the ratio of polytypic plasma cells to total plasma cells.
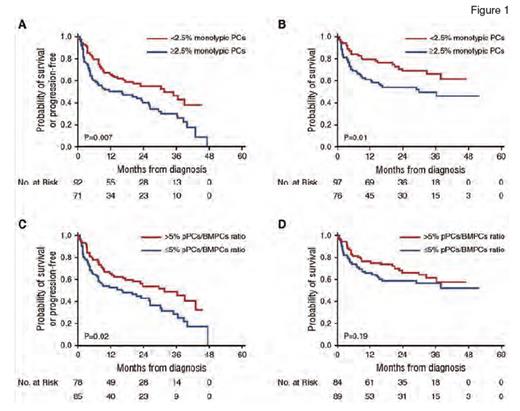
Survival Curves From Diagnosis Using the Kaplan-Meier Method for Patients With Multiparametric Flow Cytometry Immunophenotyping at Diagnosis. (A) Progression-free survival (PFS) stratified by monotypic plasma cells (PCs) cutoff at 2.5%. (B) Overall survival stratified by monotypic PCs cutoff at 2.5%. (C) PFS stratified by polytypic PCs (pPCs)/bone marrow PCs (BMPCs) ratio cutoff at 5%. (D) Overall survival stratified by pPCs/BMPCs ratio cutoff at 5%.
Survival Curves From Diagnosis Using the Kaplan-Meier Method for Patients With Multiparametric Flow Cytometry Immunophenotyping at Diagnosis. (A) Progression-free survival (PFS) stratified by monotypic plasma cells (PCs) cutoff at 2.5%. (B) Overall survival stratified by monotypic PCs cutoff at 2.5%. (C) PFS stratified by polytypic PCs (pPCs)/bone marrow PCs (BMPCs) ratio cutoff at 5%. (D) Overall survival stratified by pPCs/BMPCs ratio cutoff at 5%.
At diagnosis, those with higher numbers of monotypic plasma cells had inferior progression-free survival (PFS) as well as overall survival (OS), using a cutoff of ≥ 2.5 percent for monotypic plasma cells. Interestingly, morphologic enumeration of plasma cells did not demonstrate a similar difference in PFS or OS, using a cutoff of 10 percent or greater (two-year PFS of 50% vs. 48% in those with plasma cells <10%; p=0.9; 2-year OS of 65% vs. 60% in those with <10% plasma cells; p=0.9). On the other hand, patients with reduced proportion of polytypic plasma cells by flow cytometry (i.e., polytypic plasma cells/total plasma cell ratio of ≤5%) had shorter PFS, but no significant difference in OS. These findings held true in both univariate and multivariate analyses (Figure 1).
When the authors stratified those who underwent autologous stem cell transplantation (ASCT; N=108) from those who did not (N=65), higher monotypic plasma cells (≥2.5%) and a lower proportion of polytypic plasma cells (≤5%) were associated with a worse PFS and OS only in the non-ASCT group.
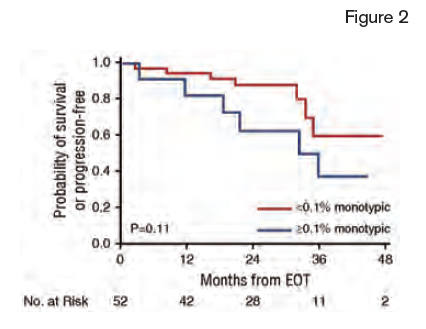
Survival Curves From the End of the First Line of Treatment (EOT) for Patients Without Evidence for Progression at EOT. Progression-free survival stratified by monotypic plasma cells at 0.1% cutoff.
Survival Curves From the End of the First Line of Treatment (EOT) for Patients Without Evidence for Progression at EOT. Progression-free survival stratified by monotypic plasma cells at 0.1% cutoff.
Of the 82 patients analyzed at the end of the first line of treatment, 69 (84%) had undergone ASCT, 10 (12%) received bortezomib-based therapy, and three (4%) received melphalan-based therapy. Patients with ≥0.1 percent monotypic plasma cells had a shorter PFS (2-year PFS 31% vs. 87%, p<0.0001), and OS (2-year OS 87% vs. 98%; p=0.02) compared to those with <0.1 percent monoclonal plasma cells. They also conducted a subgroup analysis of patients who had a very good partial response or better, by standard hematologic criteria (dFLC <40 mg/L). In this subgroup, flow cytometric quantification of monotypic plasma cells had added predicted value; those with ≥0.1 percent monotypic plasma cells had a higher rate of progression versus those with <0.1 percent monotypic plasma cells (54% vs. 17%, p=0.1; Figure 2).
In Brief
This study demonstrates the value of using multiparametric flow cytometry in patients with AL amyloidosis, both at diagnosis and at the end of treatment. Increased percentage of monotypic plasma cells and decreased proportion of polytypic plasma cells are indicative of a worse prognosis and of shortened overall survival in patients who do not undergo ASCT. After therapy, flow cytometric enumeration of monotypic plasma cells may help further risk stratify those with an apparent very good hematologic response.
References
Competing Interests
Dr. George indicated no relevant conflicts of interest.