Study Title:
Study of Biomarker-Based Treatment of Acute Myeloid Leukemia
ClinicalTrials.gov Identifier:
Sponsor:
Leukemia & Lymphoma Society
Participating Centers:
Memorial Sloan Kettering Cancer Center, Ohio State University Comprehensive Cancer Center, Oregon Health & Science University Knight Cancer Institute, Dana-Farber Cancer Institute and Massachusetts General Hospital Cancer Center with anticipated expansion to other centers
Accrual Goal:
500 patients
Study Design:
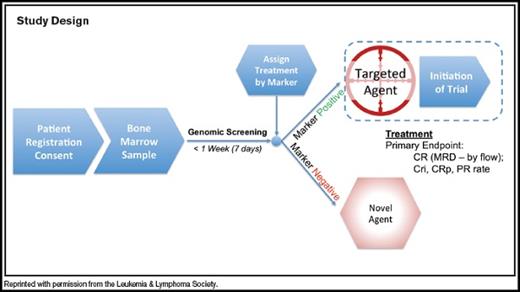
This is an umbrella phase Ib/II protocol designed to offer targeted therapy to older adults with newly diagnosed acute myeloid leukemia (AML) based on their genetic characteristics. The protocol consists of an initial seven-day screening phase, followed by assignment to targeted therapy on one of several companion biomarker-based treatment protocols. After study enrollment, bone marrow is sent to a dedicated laboratories for genomics screening while patients receive supportive therapy. Results are returned within seven days, and patients are subsequently assigned to one of several separate substudies using novel therapy alone, or in combination with conventional “7+3” (daunorubicin plus cytarabine) chemotherapy or hypomethylating agents, based on their target profile (Figure). The Beat AML Master Trial will start with four treatment arms but is envisioned to expand to as many as 10 to 12 treatment arms investigating novel molecularly or immunologically targeted therapies for AML in the future. Screened patients without an identified targetable lesion are eligible to receive novel therapy on a marker-negative substudy. Biopharmaceutical companies are participating in the trial and providing the investigational agents for this study. The first investigational agents include AG-221, an IDH2 inhibitor; entospletinib, a spleen tyrosine kinase (SYK) inhibitor; samalizumab, a monoclonal anti-CD200 antibody; and BI 836858, a monocolonal anti-CD33 antibody.
Study Design Diagram. Reprinted with permission from the Leukemia & Lymphoma Society.
Study Design Diagram. Reprinted with permission from the Leukemia & Lymphoma Society.
Patients aged 60 years and older with newly diagnosed AML are eligible for this study and can receive targeted therapy for as long as they are responding, or alternatively, they can undergo hematopoietic stem cell transplantation if they achieve favorable responses. Primary outcome measures for this trial are determining the feasibility of completing all molecular, genetic, immunophenotypic, and/or biochemical studies needed to classify disease subtype and assign therapy within seven days. The clinical response rate to each novel therapy is also a primary outcome measure. Patient-reported outcomes will be included as an important exploratory objective.
Rationale:
Despite the use of intensive cytotoxic chemotherapy and hematopoietic stem cell transplantation during the past 40 years, the prognosis of older AML patients has not improved and has lagged behind that observed in younger patients.1 Historically, AML has been classified by routine cytogenetics and screening for select chromosomal alterations. Recently, however, a growing number of genetic alternations have been identified, highlighting the disease heterogeneity and the challenges in developing a common treatment approach for all patients.2
Moreover, emerging evidence suggests that molecularly targeted therapies, such as FLT3 or IDH inhibitors, may be effective in distinct AML subtypes, and this has provided the rationale for the personalized medicine approach in this trial. The trial will be limited to older adults as this is a group that has historically fared poorly with traditional cytotoxic chemotherapy.
Comment:
This trial is a unique collaboration between patients, researchers, pharmaceutical companies, laboratories, and regulatory agencies to develop a precision medicine approach for the treatment of AML. If successful, this trial could facilitate the approval of new drugs of promise. This trial will also capture patient-related outcomes, which will provide an essential perspective on this novel treatment approach. Challenges will include obtaining the information needed for treatment assignment in a short window of time in a multicenter setting and the feasibility of a seven-day waiting period before starting definitive therapy. However, this study represents an innovative and very exciting treatment paradigm in a disease setting where effective targeted therapy requires close alignment with disease subtype.
Note. ASH has partnered with the Leukemia & Lymphoma Society to help spread the word about this pivotal trial.
References
Competing Interests
Dr. Raetz and Dr. Kovacsovics indicated no relevant conflicts of interest.