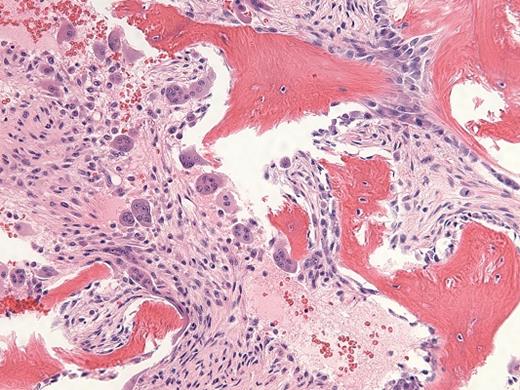
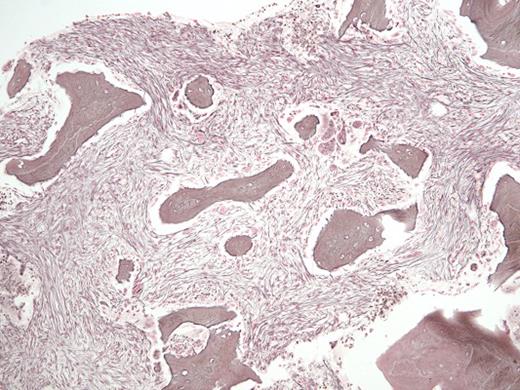
A 29-year-old man with a history of recurrent necrotizing pancreatitis and end-stage renal disease on intermittent hemodialysis was referred for outpatient consultation. He has no history of bruising or bleeding but was recently found to have pancytopenia. A CBC revealed a white blood cell count of 1.6 × 109/L, absolute neutrophil count of 0.4 × 109/L, hemoglobin of 9.1 g/dL, and platelet count of 83 × 109/L. A bone marrow biopsy (Figure 1, hematoxylin and eosin stain; Figure 2, reticulin stain) is shown in the images.
Hematoxylin and Eosin Stain. Hematoxylin and eosin stain in a 29-year-old man with a history of recurrent necrotizing pancreatitis and end-stage renal disease.
Hematoxylin and Eosin Stain. Hematoxylin and eosin stain in a 29-year-old man with a history of recurrent necrotizing pancreatitis and end-stage renal disease.
Reticulin Stain. Reticulin stain in a 29-year-old man with a history of recurrent necrotizing pancreatitis and end-stage renal disease.
Reticulin Stain. Reticulin stain in a 29-year-old man with a history of recurrent necrotizing pancreatitis and end-stage renal disease.
A. Primary myelofibrosis
B. Osteitis fibrosa
C. Gray platelet syndrome
D. Systemic lupus erythematosus
Sorry, that was not the preferred response.
Correct!
The H&E stained bone marrow slide demonstrates increased numbers of immature-appearing osteoblasts seen at sites of osteoid deposition (upper right; normal ~40% of trabecular bone surface area), opposite increased numbers of multinucleate osteoclasts seen at sites of resorptive surfaces (center; normal <2% of trabecular bone surface area). The high dynamic bone turnover rate created by these increased numbers of cells leads to thin and scalloped appearing trabecular bone, with increased amounts of immature woven osteoid seen on trichrome staining (not shown). The reticulin stained bone marrow slide demonstrates areas of diffuse, dense increases in reticulin fibers with extensive intersections and coarse bundles of collagen, consistent with MF-3 grade fibrosis (upper right); interspersed with areas of less dense fiber deposition consistent with MF-2 grade fibrosis (center). The remainder of his laboratory testing was unremarkable, including serum iron and ferritin levels, serum and urine paraprotein studies, inflammatory markers, thyroid hormone levels, and vitamin B12 and folate levels. Subsequent chromosomal analysis revealed a normal 46, XY karyotype, and testing for the JAK2 V617F, BCR-ABL1, MPL W515 L/K, and CALR exon 9 mutations were all negative.
Corroborative history provided by his dialysis center indicated that he was prescribed the calcimimetic agent cinacalcet, which had been unable to be appropriately titrated in response to monthly intact parathyroid hormone (iPTH) monitoring to manage his secondary hyperparathyroidism (see Table 1). The persistently elevated iPTH and alkaline phosphatase (ALP) levels, in addition to the extensive dynamic bone turnover and marrow fibrosis seen in the absence of additional molecular or genetic evidence of a myeloproliferative neoplasm are consistent with a diagnosis of high-turnover renal osteodystrophy, of the osteitis fibrosa subtype.1,2 In high-turnover bone disease, the exact pathophysiology behind the development of fibrosis in the peritrabecular bone marrow space has remained elusive. Normal osteoblast differentiation is prevented by secondary hyperparathyroidism seen in association with osteitis fibrosa, due in part to decreased levels of one of the bone morphogenetic proteins (BMP-7) which is expressed at high levels in the normal functioning kidney, and induces osteoblast growth and differentiation.3 The absence of BMP-7 in patients with markedly decreased renal mass may underlie the abnormal development of osteoblasts into more fibroblast-like cells, thereby leading to collagen deposition and osteitis fibrosa. This was supported by an animal model of chronic renal failure and secondary hyperparathyroidism, in which the administration of BMP-7 prevented peritrabecular fibrosis, induced normal osteoblast development, and decreased bone resorption.4
The diagnosis of overt primary myelofibrosis according to the revised 2016 WHO criteria requires 3 major criteria and and at least one of four minor criteria. The three major criteria include: (1) megakaryocytic proliferation and atypia, with or without reticulin and/or collagen fibrosis; (2) criteria for polycythemia vera (PV), essential thrombocythemia (ET), chronic myeloid leukemia (CML), myelodysplastic syndrome (MDS), or other myeloid neoplasm are not met; and (3) demonstration of a JAK2, CALR, or MPL mutation or another clonal marker (ASXL1, EZH2, TET2, IDH1/IDH2, SRSF2, or SR3B1 mutation) or no identifiable cause of reactive fibrosis. The patient does not meet these criteria.
Gray platelet syndrome (GPS) is a rare inherited bleeding disorder characterized by macrothrombocytopenia and absence of platelet α-granules resulting in pale and hypogranulated gray platelets on peripheral blood smear, as well as myelofibrosis and splenomegaly. Mutations in the genes GFI1B and NBEAL2 have been identified in autosomal dominant and autosomal recessive forms of GPS, respectively. Systemic lupus erythematosus can affect the bone marrow in a myriad of ways, including bone marrow failure (eg. aplastic anemia), macrophage activation syndrome (eg. hemophagocytic lymphohistiocytosis/ HLH), lymphoproliferative disease, as well as myelofibrosis. However, without any additional systemic symptoms consistent with autoimmune disease, this remains an unlikely cause of this patient’s myelofibrosis.
| Date | WBC Count (x109/L) | Hemoglobin (g/dL) | Platelet Count (x109/L) | ALP (U/L) | iPTH (pg/mL) |
| 12-2012 | 6.7 (ANC 3.0) | 8.8 | 180 | 69 | 72.76 |
| 03-2015 | 372 | 603.8 | |||
| 11-2015 | 557 | 1640 | |||
| 03-2016 | 2.3 | 9.0 | 112 | 721 | 665 |
| 04-2016 | 938 | ||||
| 05-2016 | 2.7 | 12.6 | 106 | 881 | 1577 |
| 06-2016 | 2547 | ||||
| 07-2016 | 4.4 | 12.9 | 117 | 1340 | |
| 08-2016 | 1.6 (ANC 0.4) | 9.1 (Retic 0.3%) | 83 | 644 | 1632 |
| Date | WBC Count (x109/L) | Hemoglobin (g/dL) | Platelet Count (x109/L) | ALP (U/L) | iPTH (pg/mL) |
| 12-2012 | 6.7 (ANC 3.0) | 8.8 | 180 | 69 | 72.76 |
| 03-2015 | 372 | 603.8 | |||
| 11-2015 | 557 | 1640 | |||
| 03-2016 | 2.3 | 9.0 | 112 | 721 | 665 |
| 04-2016 | 938 | ||||
| 05-2016 | 2.7 | 12.6 | 106 | 881 | 1577 |
| 06-2016 | 2547 | ||||
| 07-2016 | 4.4 | 12.9 | 117 | 1340 | |
| 08-2016 | 1.6 (ANC 0.4) | 9.1 (Retic 0.3%) | 83 | 644 | 1632 |
References
References
The authors indicated no relevant conflicts of interest.