This year marked the first year of fiscal funding for the U.S. Precision Medicine Initiative, with $215 million allotted to support this initiative, $70 million of which was specifically allocated to the National Cancer Institute to lead efforts in cancer genomics as part of the “Precision Medicine Initiative for Oncology.” Coincident with the announcement of this initiative, ASH launched a task force focusing on precision medicine in 2015, aimed at evaluating and advancing the use of this emerging approach for malignant and nonmalignant hematology.
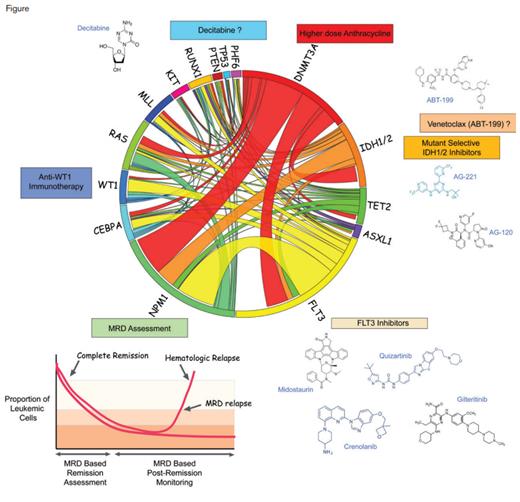
Therapeutic and Diagnostic Interventions Based on Molecular Alterations in Acute Myeloid Leukemia (AML). Circos plot illustrating common molecular alterations in de novo AML in adults between the ages of 16-60 years of age (center). Molecularly targeted therapies based on the presence of certain mutations are shown around the Circos plot and include mutant-selective IDH1/2 inhibitors and FLT3 inhibitors and potentially the use of venetoclax and decitabine for IDH1/2 mutant and TP53-mutant AML, respectively. In addition to the use of mutations as therapeutic targets, certain mutations (such as mutant NPM1) may also be followed for minimal residual disease (MRD) assesment in AML. Not shown here are splicing factor mutations, which are more frequent in secondary AML and older patients and are being tested for selective sensitivity to spliceosome targeting agents.
Therapeutic and Diagnostic Interventions Based on Molecular Alterations in Acute Myeloid Leukemia (AML). Circos plot illustrating common molecular alterations in de novo AML in adults between the ages of 16-60 years of age (center). Molecularly targeted therapies based on the presence of certain mutations are shown around the Circos plot and include mutant-selective IDH1/2 inhibitors and FLT3 inhibitors and potentially the use of venetoclax and decitabine for IDH1/2 mutant and TP53-mutant AML, respectively. In addition to the use of mutations as therapeutic targets, certain mutations (such as mutant NPM1) may also be followed for minimal residual disease (MRD) assesment in AML. Not shown here are splicing factor mutations, which are more frequent in secondary AML and older patients and are being tested for selective sensitivity to spliceosome targeting agents.
“Precision medicine” is widely defined as the use of a patient’s molecular characteristics (at the genetic, transcriptomic, and/or proteomic level) to prevent, diagnose, and/or treat disease. Given the relative ease of access of blood and bone marrow cells, the field of hematology has historically been at the vanguard of the use of molecular characterization for clinical practice. At the same time, there are unfortunately several examples of hematologic disorders where outcomes have not improved despite decades of advances in molecular diagnostics. For example, it is widely cited that there have been no significant advances in outcome for patients with acute myeloid leukemia (AML) in 40 years, despite detailed knowledge of the cytogenetic and molecular markers that predict prognosis. Given these challenges, we present here a year-end critical appraisal of the progress and challenges in the use of personalized medicine to improve outcomes in AML.
Currently, treatment decisions for the majority of patients with AML depend on age and cytogenetic characteristics, and only occasionally on the use of a limited number of molecular alterations at diagnosis (mutations in FLT3, NPM1, and CEBPA, and possibly IDH1/2 mutations). However, there has been increasing appreciation of the molecular heterogeneity of AML and the potential utility of considering larger numbers of molecular alterations at diagnosis to refine prognosis. To this end, one of the largest studies to correlate mutations with outcome in AML was published this year1 and suggested the existence of additional molecular subgroups of AML that have not been widely appreciated in the past. Through sequencing 111 genes across 1,540 AML patients from three different German AML Study Group trials, Dr. Elli Papaemmanuil and colleagues suggested the existence of three novel molecularly defined subgroups of AML, in addition to currently defined AML subgroups, as follows1 : AML with mutations in genes encoding chromatin and/or RNA-splicing factors; AML with TP53 mutations and/or chromosomal aneuploidies; and AML with IDH2 R172 mutations. The adverse prognosis associated with the “chromatin-spliceosome” and “TP53-aneuploidy” AML groups further suggests the need to include characterization of mutations in genes in these pathways into clinical diagnostics for AML, a practice not widely adopted today for most patients with AML, even in the United States.
Despite the advances made in the aforementioned study, the complexities of molecular alterations and their interactions in AML remain a major challenge to implement in clinical use. To this end, exciting advances have been made in the use of minimal residual disease (MRD) monitoring in AML. One example of this was an illustrative study of the power of MRD monitoring by quantifying NPM1-mutated transcripts for patients with NPM1-mutated AML.2 Despite the numerous potential mutations that may co-occur with the NPM1 mutation in AML, monitoring for the presence of this mutation alone was the single most powerful method for predicting disease outcome. These data suggest that outcome in AML may be better predicted by MRD monitoring than by extensive molecular profiling at diagnosis. In addition to technical issues related to specific mutations and/or flow-cytometric–based markers to be followed for MRD assessment, whether or not MRD monitoring will actually improve outcomes in AML remains to be seen.
One of the unfortunate paradoxes in AML has been that despite detailed knowledge of the molecular pathogenesis of the disease, effective molecularly targeted therapies in AML have been challenging to develop. The use of mutant-selective IDH1/2 inhibitors remains exciting in this regard, and updates from the 2016 ASH Annual Meeting reveal the potential utility of mutant IDH2 inhibition with AG-221 for IDH2-mutant patients with myelodysplastic syndromes (MDS) specifically.3 Additionally, a novel, clinical compound aimed at targeting spliceosomal mutant cancers (H3B-8800) was described at the 2016 ASH meeting,4 and a phase I trial of this compound has now been initiated at multiple centers in the United States for patients with nearly any refractory myeloid malignancy (clinicaltrials.gov, NCT02841540). Finally, it has long been suspected that one of the challenges in the development of novel molecularly targeted therapies in AML has been the practice of testing novel agents as sole therapies in patients with relapsed or refractory disease, sometimes without requirement of the molecular alteration targeted by the compound for enrollment. To this end, in 2016, the Leukemia & Lymphoma Society announced their sponsorship of a multisite, multitreatment, multiarm trial for newly diagnosed AML patients aged 60 years and older — the “Beat AML Master Trial” (clinicaltrials.gov, NCT02927106). This trial will enroll newly diagnosed patients with AML and provide rapid genomic screening within one week. Based on the results, individual patients will be assigned to receive personalized therapy on one of several substudies of the protocol. The targeted therapies included on the study currently include: Alexion’s CD200 antibody, samalizumab; Celgene’s IDH2 inhibitor, AG-221; Gilead’s Syk inhibitor, entospletinib; and Boehringer Ingelheim’s CD33 monoclonal antibody, BI-836858, with more arms to open and potentially include combination therapies.
With the culmination of a year’s worth of advances in understanding the molecular underpinnings of AML and its response to conventional therapy, we look forward to seeing these translate into new therapies and better outcomes for patients in the coming year. For example, given the U.S. Food and Drug Administration’s breakthrough therapy designation assigned for the FLT3 inhibitor, midostaurin, we are hopeful to see the first targeted therapy approved for AML.
References
Competing Interests
Dr. Taylor and Dr. Abdel-Wahab indicated no relevant conflicts of interest.