The first bone marrow stem cell transplantations were attempted almost 60 years ago. Since then, this procedure has continued to expand in not only the number of patients, but also an ever-growing list of diseases that have the potential to be treated or even cured.
Early on, the considerations for bone marrow transplant were largely driven by the societal concerns of the time. In 1956, Dr. E. Donnall Thomas reported1 some of the first attempted bone marrow transplants from a variety of cellular sources. In his conclusion, Dr. Thomas stated that:
“In an atomic age, with reactor accidents, not to mention stupidities with bombs, somebody is going to get more radiation than is good for him. If infusion of marrow can induce recovery in a mouse or monkey after lethal radiation, one had best be prepared with this form of treatment in man.”
Radiation accidents and nuclear warfare were the prevailing concern, and bone marrow stem cells were largely being studied as a countermeasure against lethal damage. Dr. Thomas also astutely commented, “The leukemic patients who need radiation and bone marrow… deserve immediate consideration. From helping them, one will be preparing for the atomic disaster of tomorrow.”
Remarkably, the method of conditioning a patient for bone marrow transplantation has not changed much since the original studies; doses of irradiation and/or chemotherapeutic agents are used to clear the marrow space of resident hematopoietic and immune cells, allowing the new cells to engraft in the limited niches available. While this is certainly acceptable after a radiation-related mass-casualty event, or after the treatment of an aggressive malignant disease, using this “bomb” approach results in toxicities that normally limit the use of stem cell transplantation to only a fraction of the patients who could potentially benefit.
With the emergence of developing gene therapies for nonmalignant disease, and increasing evidence of the potential curative option for many other diseases, a less toxic, more targeted conditioning approach is needed to broadly expand transplantation to these patient groups. Recently, two independent teams have published conditioning methods based on antibody targeting.
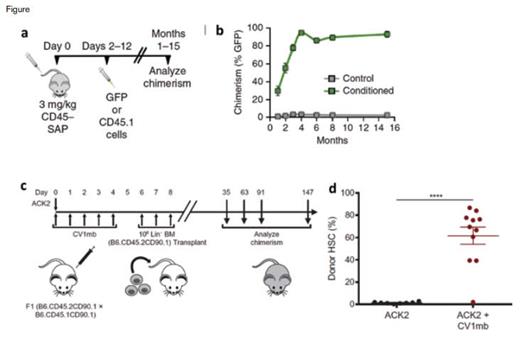
Dr. Rahul Palchaudhuri and colleagues2 report on the use of an immunotoxin, created by conjugating a CD45 antibody with saporin, which they term CD45-SAP (Figure, a and b). Saporin is a toxin in the ricin family, but unlike ricin it lacks the ability to enter a cell on its own, and instead must be conjugated to an antibody or ligand capable of internalization. Given that CD45 is expressed exclusively in the hematopoietic system, this targeted approach allowed for depletion of the bone marrow space, without unintended off-target toxicities. In mice, a single treatment of CD45-SAP followed by transplantation of 10 million whole bone marrow cells (roughly 2% of the total marrow of a mouse) resulted in 94 percent chimerism and complete, multilineage engraftment. Remarkably, when the same dose of CD45-SAP was given to mice, with no subsequent stem cell transplant, all of the mice recovered, demonstrating that the CD45-SAP is capable of conditioning to allow for high levels of engraftment, without being permanently myeloablative. Analysis of the bone marrow vasculature demonstrated no damage, in contrast to sublethal irradiation, suggesting preservation of the hematopoietic niche. Additionally, much of the thymic tissue was preserved, with a demonstrated increase in de novo T cell production. Using a mouse model of sickle cell anemia, the researchers also demonstrated that CD45-SAP was able to effectively condition, and a subsequent transplantation resulted in correction of the disease phenotype.
While CD45 is exclusively expressed in hematopoietic cells, it is not restricted to just stem and progenitor cells, but is widely expressed across all of the nucleated blood cells. In the autologous gene therapy setting, it may be more desirable to just specifically remove stem and progenitor cells, while preserving immune cell function, given the reduced chance of graft rejection since the transplanted stem cells would be of host origin. To specifically target stem and progenitor cells, a prior study had focused on antibody targeting of the c-kit receptor with the antibody ACK2.3 This report showed the ability to achieve modest amounts of engraftment after transplantation with high doses of purified stem cells. Surprisingly, this conditioning method only worked in immunocompromised mice and did not work in immunocompetent animals.
Protocol Schematics. a) Schematic of CD45-SAP conditioning protocol. Mice were treated with 3 mg/kg CD45-SAP and transplanted with 107 bone marrow cells two to 12 days later. b) Long-term assessment of peripheral blood chimerism after transplantation eight days following CD45-SAP conditioning. c) Schematic of combination ACK2 and CV1mb conditioning protocol. Mice were treated once with 500 μg ACK2 and 500 μg CV1mb for five days. A day later, 1 million lineage-depleted bone marrow cells were transplanted. d) Frequency of donor-derived hematopoietic stem cells 24 weeks after transplantation.
Protocol Schematics. a) Schematic of CD45-SAP conditioning protocol. Mice were treated with 3 mg/kg CD45-SAP and transplanted with 107 bone marrow cells two to 12 days later. b) Long-term assessment of peripheral blood chimerism after transplantation eight days following CD45-SAP conditioning. c) Schematic of combination ACK2 and CV1mb conditioning protocol. Mice were treated once with 500 μg ACK2 and 500 μg CV1mb for five days. A day later, 1 million lineage-depleted bone marrow cells were transplanted. d) Frequency of donor-derived hematopoietic stem cells 24 weeks after transplantation.
To boost the effectiveness of c-kit targeting and to apply the conditioning method to immunocompetent mice, Dr. Akanksha Chhabra and colleagues4 recently coupled the ACK2 antibody with antagonism of CD47 (Figure, c and d). Acting as a “don’t eat me” signal, CD47 is present on stem cells and a wide variety of other immune cells and prevents phagocytosis and antigen presentation from macrophages and dendritic cells.5 Prior work in tumor biology has shown that blockade of CD47 enhances tumor antibody opsonization, so the authors reasoned that similar enhancements could be made with the c-kit targeting antibody. When ACK2 was given on its own in immunocompetent C57Bl/6 mice there was no depletion of stem cells, as was previously shown. However, the combination of ACK2 with the mouse-specific CD47 antagonist CV1mb resulted in robust depletion of phenotypically defined stem and progenitor cells and a marked clearance of the bone marrow space. When these mice then received transplants with stem and progenitor cell–enriched bone marrow for three consecutive days after the conditioning treatment, approximately 60 percent chimerism was achieved 20 weeks after transplantation, demonstrating that the combined treatment approach effectively increased the efficacy of c-kit targeting in immunocompetent animals. In an effort to expand this approach to a broader group of patients, who would likely receive an allogeneic graft, the researchers used a mouse model of minor histocompatibility mismatch. When Balb/c mice were treated with the ACK2/anti-CD47 regimen, along with a regimen of anti-CD4/CD8 antibodies to deplete T cells, they were able to achieve approximately 20 percent stem cell chimerism after a purified stem cell transplant from the mismatched B10D2 mice.
These new, targeted conditioning approaches come at an exciting time for stem cell transplantation, in which the potential patient pool could be remarkably expanded with the advent of new gene therapy and editing strategies. Unlike the bomb-like approaches developed since the procedure’s beginnings, these SWAT teams of specific antibodies may allow for conditioning regimens that are not only nongenotoxic, but that may allow for almost near-outpatient treatment.
References
Competing Interests
Dr. Jonathan Hoggatt is an equity stake holder in Magenta Therapeutics.