Progress in the treatment of acute lymphocytic leukemia (ALL) in adults has lagged behind that in children, with cure rates of approximately 40 to 50 percent. Outcomes following relapse are dismal, despite the delivery of intensive chemotherapy with or without hematopoietic stem cell transplantation (HSCT), and the limits of tolerability have been reached with conventional cytotoxic therapies. The development of alternative treatment approaches has accordingly been a high priority, and several promising new immune therapies have been developed recently.1-3 Adding to this experience, Dr. Hagop M. Kantarjian and colleagues have reported their findings from a trial investigating inotuzumab ozogamicin, a humanized anti-CD22 monoclonal antibody bound to calicheamicin, in adults with relapsed or refractory ALL.
Treatment Schema. Inotuzumab ozogamicin dose reduced to 1.5 mg/m2/cycle once complete remission/complete remission with incomplete hematologic recovery was achieved. Abbreviations: ALL, acute lymphocytic leukemia; FLAG, fludarabine, cytarabine, and granulocyte colony-stimulating factor. Adapted with permission from N Engl J Med. 2016;375:740-753.
Treatment Schema. Inotuzumab ozogamicin dose reduced to 1.5 mg/m2/cycle once complete remission/complete remission with incomplete hematologic recovery was achieved. Abbreviations: ALL, acute lymphocytic leukemia; FLAG, fludarabine, cytarabine, and granulocyte colony-stimulating factor. Adapted with permission from N Engl J Med. 2016;375:740-753.
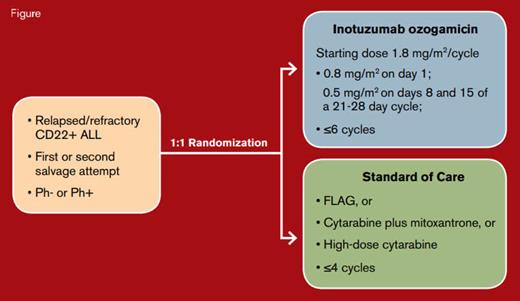
Investigators from 18 international sites conducted the INO-VATE phase III study in adults 18 years or older with relapsed or refractory CD22-positive B-cell ALL undergoing first or second salvage therapy. Patients were randomly assigned 1:1 to treatment with inotuzumab ozogamicin versus one of three standard cytotoxic chemotherapy regimens of investigator choice (Figure). Patients aged 18 to 79 years (median, 47 years) participated in this trial. Inotuzumab ozogamicin was administered weekly for three doses per 21- to 28-day cycle for up to six cycles. Those assigned to conventional chemotherapy received up to four cycles of standard chemotherapy, and any patient in either treatment group who achieved complete remission (CR) could undergo HSCT.
The primary endpoints for this study were rates of CR, which included CR with incomplete hematologic recovery (CRi), and overall survival. Patients receiving inotuzumab ozogamicin had a significant improvement in several outcome measures compared with the standard chemotherapy group. Among the first 218 patients comprising the remission analysis population (intention-to-treat), the CR rates for the inotuzumab ozogamicin group were 80.7 percent versus 29.4 percent, P<0.001 in the standard therapy group. Similar significant improvements in CR rates with inotuzumab ozogamicin were also observed in an as-treated analysis. Among those achieving CR, a significantly higher percentage of patients in the inotuzumab ozogamicin group had a minimal residual disease (MRD) burden below a threshold of 0.01 percent by flow cytometry (78.4% vs. 28.1%, p<0.001), and a significantly higher percentage proceeded to HSCT. The duration of remission, progression-free survival, and overall survival was also longer with inotuzumab ozogamicin compared with standard chemotherapy in the 326 patients included in the intention-to-treat survival analysis. The rate of two-year overall survival was 23 percent in the inotuzumab ozogamicin group and 10 percent in the standard therapy group. Overall, inotuzumab ozogamicin was well tolerated. The only significant difference in toxicity was a higher rate of hepatic adverse events. Veno-occlusive disease of the liver was more common in the inotuzumab ozogamicin group versus the standard chemotherapy group (11% vs. 1%), and the risk was highest among patients who received the drug prior to or following HSCT with a dual-alkylator conditioning regimen. The toxicities of inotuzumab ozogamicin were otherwise comparable to those observed with standard chemotherapy.
In Brief
This article describes a highly promising new monoclonal antibody therapy for CD22-positive B-cell ALL. The significant improvement in CR rates with inotuzumab ozogamicin is a key finding because attaining CR is generally viewed as prerequisite to HSCT, which is currently the only curative modality for relapsed and refractory ALL in adults. Furthermore, the toxicity profile of inotuzumab ozogamicin compared favorably to that of intensive cytotoxic chemotherapy with fewer off-target effects, with the exception of hepatic veno-occlusive disease. Although not formally assessed in this study, outpatient delivery is another appealing benefit of this regimen. This study opens new avenues for investigation of inotuzumab ozogamicin in combination with chemotherapy and among expanded patient populations.
References
Competing Interests
Dr. Raetz indicated no relevant conflicts of interest.