Study Title:
A Phase 2 Study of the JAK1/JAK2 Inhibitor Ruxolitinib with Chemotherapy in Children with De Novo High-Risk CRLF2-Rearranged and/or JAK Pathway–Mutant Acute Lymphoblastic Leukemia
ClinicalTrials.gov Identifier:
Pending
Sponsor:
Incyte and National Cancer Institute
Coordinator:
Children’s Oncology Group
Participating Centers:
Multiple sites in the United States and other countries
Accrual Goal:
Up to 170 patients; in Part 2, Cohorts A and B will accrue a target of 42 subjects each
Raetz 4/25/16
Study Design:
This two-part trial will first evaluate the safety and tolerability of, and define the recommended dose of, the Janus kinase (JAK) inhibitor ruxolitinib in combination with a multiagent chemotherapy platform that is standard for National Cancer Institute (NCI) high-risk (HR) B-cell acute lymphoblastic leukemia (B-ALL). Part 2 of the study will test whether the addition of the ruxolitinib (using the dose defined in part 1), to a standard multiagent chemotherapy platform improves the three-year event-free survival (EFS) for children, adolescents, and young adults with newly diagnosed HR B-ALL with cytokine receptor–like factor 2 rearrangements (CRLF2-R) and/or other JAK pathway alterations compared with historical controls treated with an identical chemotherapy regimen without ruxolitinib.
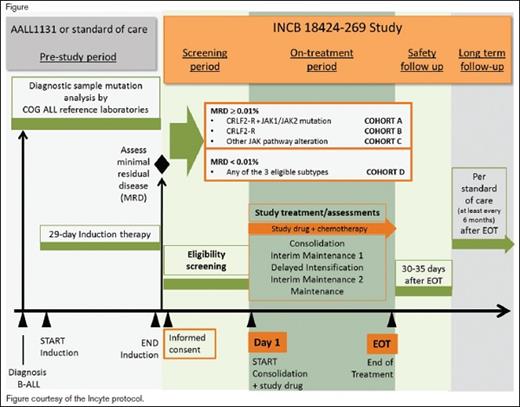
Patients aged one to 21 years who have newly diagnosed B-ALL and are also confirmed to have Ph-like ALL with CRLF2-R and/or JAK pathway mutations and HR features (age 10 years or older at diagnosis and/or initial white blood cell counts of 50 × 109/L or greater and/or central nervous system leukemia), are eligible. All NCI HR patients will undergo screening for Ph-like features during the first month of a standard four-drug induction phase with simultaneous confirmatory testing for defined genetic lesions. Patients will also undergo routine disease response assessment at the end of induction, which will include measurement of minimal residual disease (MRD) by flow cytometry on day 29. Patients who are confirmed during induction to have CRLF2-R and/or JAK pathway alterations are eligible for entry into the trial after the completion of standard induction therapy. Therapy on the trial will commence with the consolidation phase, which will use a modified augmented Berlin-Frankfurt-Munster (BFM) backbone. Four cohorts of patients will be studied on the trial: 1) cohort A, CRLF2-R and JAK-mutant with end induction MRD+ (≥ 0.01% by flow cytometry); 2) cohort B, CRLF2-R and JAK-wild-type with end induction MRD+; 3) cohort C, other alterations involving the JAK pathway (e.g., JAK2 fusions, EPO receptor fusions, SH2B3 deletions, IL7RA mutations) and MRD+; 4) cohort D, subjects otherwise eligible for cohorts A, B, or C and MRD–. After entry on this trial, patients will receive ruxolitinib in combination with standard post-induction phases of chemotherapy for NCI HR B-ALL.
Primary outcomes for part 1 include the determination of safety and tolerability of the combination treatment through assessment of adverse events (AEs) and changes in safety assessments, including laboratory parameters, evaluated by dose level through day 29 of delayed intensification, and determination of the recommended phase II dose (RP2D) of ruxolitinib in combination treatment. Primary outcomes for part 2 include the three-year EFS from day 1 of consolidation for subjects in cohorts A and B beginning treatment at the RP2D. Secondary outcomes include the safety and tolerability of the combination treatment for subjects beginning treatment at the RP2D through the end of treatment.
Rationale:
Children with Ph-like ALL have high rates of relapse and poor outcomes when treated with conventional chemotherapy. While overall outcomes for children and adolescents with newly diagnosed ALL are outstanding, certain subsets have not fared well, including those with Ph-like ALL. When treated with chemotherapy alone, patients with Ph-like ALL have had five-year EFS rates of 62.6 percent vs. non-Ph-like B-ALL EFS rates of 85.8 percent; p<0.0001.1 Ph-like ALL is characterized by a gene expression signature similar to that observed in Ph+ ALL, but without the underlying BCR-ABL1 fusion. Ph-like ALL comprises 15 percent of all HR cases of B-ALL and increases in frequency with age such that nearly 30 percent of young adults with ALL harbor this phenotype. Ph-like leukemias are characterized by a variety of genetic alterations associated with activated cytokine receptor signaling. Recent studies have reported sensitivity to signal transduction inhibitors including imatinib or dasatinib that target several of the underlying gene fusions that involve ABL1, PDGFRB, and CSF1R. Rearrangements of CRLF2 are observed in half of Ph-like B-ALL cases and approximately 50 percent of these cases also have JAK mutations.
A previous phase I study in pediatric patients identified the RP2D of ruxolitinib as monotherapy to be 50 mg/m2 per dose BID; however, no leukemia patients with relevant genetic changes were treated on that study.2 Additional pre-clinical reports have shown that ruxolitinib is active in CRLF2-R ALL without and with JAK mutations, suggesting that JAK inhibition may have therapeutic efficacy in the treatment of these HR patients.3,4 Other preclinical data support an intermittent dosing schedule with periodic withdrawal of JAK inhibition, albeit in myeloproliferative neoplasms with JAK mutations.5 Given the potential concern for additive hematologic toxicity when combined with chemotherapy, this study will utilize a 14-day-on/14-day-off schedule of ruxolitinib during most phases of treatment.
Comment:
This is an exciting new study in pediatric ALL that will test the contribution of ruxolitinib to traditional chemotherapy for newly diagnosed, genetically defined subsets of patients who are known to have poor outcomes with standard therapy. It is rare that new agents are tested in newly diagnosed children, but this approach will maximize any potential signal seen using this combination in chemotherapy-naïve patients. Testing this combination in a relapsed setting may both diminish its efficacy and increase potential toxicities in heavily pretreated patients. Approximately 100 centers in the United States are planning to open this trial, which will be conducted by the Children’s Oncology Group.
References
Competing Interests
Dr. Raetz and Dr. Loh indicated no relevant conflicts of interest.