The past decade has seen remarkable improvements in therapeutic options for chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL). These include the approval of ibrutinib and idelalisib, small molecule inhibitors directed at components of the B-cell receptor pathway, and the novel CD-20 directed antibodies obinituzumab and ofatumumab. Despite impressive activity, ibrutinib and idelalisib are associated with nontrivial toxicity, including atrial fibrillation and bleeding with ibrutinib, and colitis, hepatitis, and pneumonitis with idelalisib. Additionally, patients who relapse following these therapies, including those with deletions in chromosome 17p, continue to have a poor prognosis.
CLL is characterized by increased expression of the antiapoptotic protein BCL2. Targeting BCL2 using BH3-mimetics was initially tested using the drug navitoclax. In a phase II study, approximately 35 percent of patients achieved a partial remission. The drug, however, was associated with thrombocytopenia related to on-target inhibition of Bcl-xL. The second-generation BCL2 inhibitor, venetoclax, is a more potent inhibitor of BCL2 and has less activity against Bcl-xL. Preclinical data in CLL cell lines and xenograft models demonstrated promising activity.
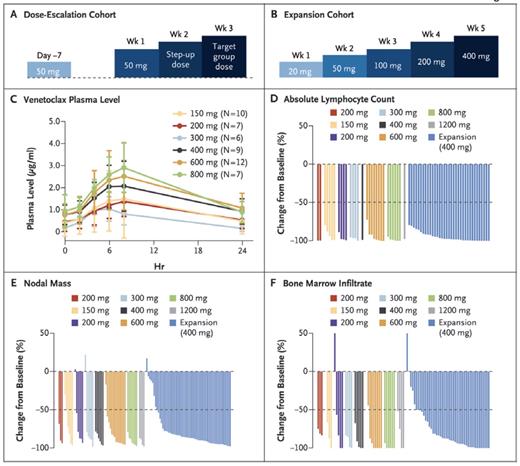
Dr. Andrew Roberts and colleagues have published results of the first phase I study of the oral drug venetoclax in relapsed or refractory CLL/SLL. Fifty-six patients were treated in the dose-escalation portion of the study (150 mg to 1,200 mg), with an expansion phase in 60 patients at a target dose of 400 mg. The patients were heavily pretreated with a median of three prior therapies (range, 1–11). Thirty and 27 percent of patients harbored deletions in chromosomes 17p and 11q, respectively, and 45 percent of patients had unmutated immunoglobulin heavy chain (IGHV).
In the initial dose escalation phase, 10 of 56 patients experienced tumor lysis syndrome (TLS), three with clinical TLS and the remaining seven with biochemical TLS. Seven patients developed TLS after the initial dose (range, 50-200 mg) and three individuals with an increase in the dose (from 150 mg to 1,200 mg). One patient experienced sudden death, and a second required hemodialysis. In total, nine patients resumed therapy. As a result of TLS, the protocol was modified such that the dose of venetoclax was slowly escalated on a weekly basis from a starting dose of 20 mg daily. Additionally, patients were hospitalized for close observation and TLS prophylaxis upon initiation of therapy, and for dose escalation in those patients at high risk for TLS. In terms of other toxicities, the most common adverse events were mild nausea and diarrhea as well as neutropenia (grade 4 in 28% of patients). The most common serious adverse events were febrile neutropenia (6%) and pneumonia (4%). A maximum tolerated dose was not identified in doses up to 1,200 mg per day.
Venetoclax Schedules, Pharmacokinetic Response, and Activity Against Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). (A) Administration schedule for the 53 patients in the dose escalation cohort. (B) Administration schedule for the 60 patients in the expansion cohort. (C) Plasma levels of venetoclax at steady state, grouped according to the dose at the time of collection. (D-F) Activity of venetoclax against CLL or SLL in blood (D), lymph nodes (E), and bone marrow (F), which are shown as normalized changes from baseline. From New England Journal of Medicine, Roberts AW et al, “Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia,” Volume 374, Page 314. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Venetoclax Schedules, Pharmacokinetic Response, and Activity Against Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). (A) Administration schedule for the 53 patients in the dose escalation cohort. (B) Administration schedule for the 60 patients in the expansion cohort. (C) Plasma levels of venetoclax at steady state, grouped according to the dose at the time of collection. (D-F) Activity of venetoclax against CLL or SLL in blood (D), lymph nodes (E), and bone marrow (F), which are shown as normalized changes from baseline. From New England Journal of Medicine, Roberts AW et al, “Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia,” Volume 374, Page 314. Copyright © 2016 Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.
Nearly all patients experienced a reduction in tumor burden (Figure). The overall (ORR) and complete response (CR) rates in all patients were 79 percent and 20 percent, respectively (77% and 30% in the dose escalation cohort and 82% and 10% in the expansion cohort). Five percent of patients had no evidence of minimal residual disease on flow cytometry. The estimated durability of response was 75% at 15 months, and response duration was longer in patients with a CR versus a partial response. For patients with 17p deletion, 71 percent of patients responded, with 16 percent achieving CR. The ORR for patients with 11q deletion was 82 percent (11% CR); the respective ORRs for IGHV unmutated versus mutated patients were, respectively, 76 percent (17% CR) versus 94 percent (29% CR). At 15 months, the progression-free survival for the 400 mg target dose groups was 69 percent. Disease progression ultimately occurred in 41 patients (35%), including Richter's transformation in 18 patients (16%).
In Brief
Venetoclax is another potent tool in our armamentarium of effective therapies for patients with CLL/SLL, including those with poor risk features. Although generally very well tolerated, the drug causes rapid destruction of tumor cells and will, therefore, need to be used with great caution, in slowly escalating doses, to prevent TLS. Future studies will, no doubt, delineate combination strategies and indicate how best to sequence therapy with other agents.
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest.