Acquired thrombotic thrombocytopenic purpura (TTP) is a thrombotic microangiopathy caused by autoantibody-mediated deficiency of the von Willebrand–cleaving protease, ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13). Reduced ADAMTS13 activity results in accumulation of ultralarge von Willebrand factor (ULvWF) multimers. Via their A1 domain, ULvWF multimers bind to glycoprotein Ib-IX-V on the surface of platelets and induce formation of platelet-rich microthrombi, leading to microvascular occlusion and end-organ ischemic injury.1 Treatment of TTP involves daily plasma exchange (PEX) to remove autoantibodies and ULvWF multimers and to replenish ADAMTS13. While PEX is effective in treating acute episodes, patients remain at risk for recurrent disease upon discontinuation of PEX due to the persistent formation of autoantibodies. Thus, immunosuppression (e.g., corticosteroids, rituximab) is often added to inhibit autoantibody production.2
Caplacizumab is a humanized single variable domain immunoglobulin that targets the A1 domain of vWF and prevents it from binding to platelets. In the phase II, single-blind, TITAN study, subjects with an acute episode of suspected acquired TTP were randomized to 10 mg/d caplacizumab subcutaneously or matching placebo during plasma exchange and for 30 days afterward. Patients with active bleeding were excluded. The primary end point was time to platelet normalization (≥ 150 × 109/L).
Seventy-five patients were assigned to the caplacizumab (n = 36) or placebo (n = 39) arms. Baseline characteristics were similar between the two groups. Two-thirds of patients were enrolled at the time of an initial episode; one-third had recurrent disease. Severe ADAMTS13 deficiency (< 10%) was documented in 77 percent of subjects at enrollment. The ADAMTS13 level at study entry was not available in 12 percent of individuals, and was ≥ 10 percent in 11 percent of subjects, suggesting that some patients had a thrombotic microangiopathy other than TTP. During PEX, 91 percent of patients received corticosteroids, and 15 percent received rituximab.
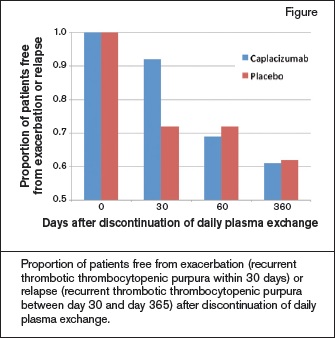
Caplacizumab shortened the median time to platelet normalization compared with placebo (39% reduction, p=0.005). Among the 69 patients in the study who did not begin PEX until after they received the first dose of study drug, the median time to response was 3.0 days (95% CI, 2.7-3.9) in the caplacizumab arm and 4.9 days (95% CI, 3.2-6.6) in the placebo group. Exacerbation (recurrent TTP within 30 days of discontinuing daily PEX) occurred in three patients in the caplacizumab group and in 11 patients in the placebo group. Relapse (recurrent TTP during the 12-month observation period but after day 30), however, occurred more commonly in patients assigned to caplacizumab (11 patients vs. 3 patients). Seven of the 11 relapses in the caplacizumab arm occurred within 10 days of stopping study drug. As might be expected for a drug that inhibits vWF function, bleeding-related adverse events were more common in caplacizumab-treated patients (54% vs. 38%). Most were mild or moderate in severity.
Proportion of patients free from exacerbation (recurrent thrombotic thrombocytopenic purpura within 30 days) or relapse (recurrent thrombotic thrombocytopenic purpura between day 30 and day 365) after discontinuation of daily plasma exchange.
Proportion of patients free from exacerbation (recurrent thrombotic thrombocytopenic purpura within 30 days) or relapse (recurrent thrombotic thrombocytopenic purpura between day 30 and day 365) after discontinuation of daily plasma exchange.
In Brief
Acquired TTP is a relapsing disease. Consistent with other published series, 29 (38.7%) of 75 patients in the TITAN study experienced an exacerbation or relapse within 12 months of discontinuing daily PEX.3 This figure may underestimate the true incidence of relapse because 1) a minority of patients in the study may have had a thrombotic microangiopathy other than acquired TTP based on non–severely deficient ADAMTS13 levels at enrollment; 2) not all patients were followed for the full 12-month study period; and 3) late relapses beyond 12 months may occur.3
PEX is a life-saving therapy for acute episodes of acquired TTP, but it does not reduce autoantibody formation or prevent exacerbation and relapse once it is discontinued. Like PEX, caplacizumab appears to be a temporizing measure but not a means of reducing recurrence. Patients randomized to caplacizumab in combination with PEX responded more quickly and had lower rates of exacerbation at 30 days than patients treated with PEX alone. However, after caplacizumab was discontinued at day 30, a “catch-up” effect was observed, with more relapses in the investigational arm than the placebo arm. By day 60, the proportion of patients free of exacerbation or relapse was no better in the investigational arm than in the placebo arm (Figure).
These findings are not unexpected based on caplacizumab’s mechanism of action, but they do call into question its potential role in the treatment of acquired TTP. Caplacizumab may be useful for managing refractory patients who do not respond to or are unable to discontinue PEX. In centers where PEX is not available, caplacizumab could also be used as a bridge, along with plasma infusion, until the patient is transferred to a center with PEX. But for most patients, it is unlikely that the benefit of shortening time to response by a few days will make up for the cost of the drug and its increased bleeding risk.
References
Competing Interests
Dr. Cuker indicated no relevant conflicts of interest.