The treatment of childhood acute lymphocytic leukemia (ALL) has been a success story, with the majority of patients now achieving cure; however, there is still a steep learning curve with regard to our understanding of how to most effectively and safely deliver conventional chemotherapeutic drugs. The bulk of childhood ALL therapy is the approximately two-year maintenance phase, the backbone of which is daily administration of 6-mercaptopurine (6MP). Questions still remain regarding how best to fine tune dosing of 6MP in individual patients and how to enhance compliance with daily oral 6MP during the several-year trajectory of an ALL treatment course. Further, despite overall high cure rates for childhood ALL, some patients develop resistance to 6MP; efforts at early detection of resistance are underway, so preemptive changes can be made to avert relapse. 6MP has been a mainstay of ALL therapy for the past six decades; however, three seminal articles published in 2015 lent critical insights into why optimal delivery of this drug is critical to the success of patient outcomes.
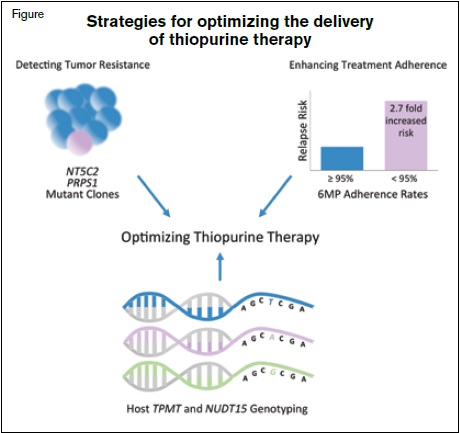
Expanding on prior studies, which have demonstrated that nonadherence to 6MP increases relapse risk,1 Dr. Smita Bhatia and colleagues reported their results from a large prospective multiyear study examining the effects of high intra-individual variability of 6MP exposure on the risk of relapse.2 The authors assessed adherence in 742 children throughout a six-month period during the maintenance phase of therapy on Children’s Oncology Group (COG) treatment regimens. The authors observed that patients with a mean 6MP adherence rate of less than 95 percent were at a 2.7-fold increased risk of relapse compared with those having 95 percent or greater adherence. Adherence was measured in this study using a special bottle cap with an electronic monitoring device to record the date and time of bottle opening for medication administration. The authors examined several other variables related to mediation adherence to determine their impact on relapse risk, including erythrocyte thioguanine (TGN) levels, 6MP dose intensity (number of prescribed 6MP doses divided by the number of planned protocol doses), thiopurine methyltransferase (TPMT) genotype, and absolute neutrophil count (ANC). Interestingly, dose intensity and TGN levels were not associated with the risk of relapse. Notably, however, among adherent patients, high intra-individual variability in TGN levels increased relapse risk by 4.4 fold. This variability in TGN levels was the result of varying 6MP dose intensity and drug treatment interruptions.
These findings raise important questions about how best to administer 6MP during ALL maintenance therapy. Presently, in COG protocols, 6MP dose adjustments are made in an effort to target an ANC range of 1 to 1.5 × 109/L, and 6MP is held when the ANC and/or platelet count are lower than 0.5 × 109/L and 50 × 109/L, respectively. Once blood counts recover, 6MP is reintroduced at a reduced dose with incremental dose increases, as tolerated. Patients tend to experience fluctuations in their blood counts that require 6MP dosing modifications, and given the association of TGN variability and relapse, this raises the question of whether modifications to current dosing guidelines should be considered in an effort to reduce drug interruptions and maintain more consistency in TGN levels.
The work by Dr. Bhatia and colleagues also ties in well with other influential reports this year describing a new host polymorphism impacting tolerance of 6MP and new discoveries on mechanisms of thiopurine resistance. While adherence to 6MP is key to minimizing relapse risk, understanding how inherited variants contribute to 6MP intolerance is also important so that consistent exposure can be maintained and excessive toxicity can be avoided. In early 2015, Dr. Jun Yang and colleagues identified a novel germline variant in the gene NUDT15, which was strongly associated with 6MP intolerance and which was most frequently found in patients of East Asian and Hispanic descent.3 The newly identified variant is hypothesized to lead to a loss-of-function phenotype for NUDT15, causing extensive DNA damage and cytotoxicity. Patients that were homozygous for the TT genotype only tolerated 8.3 percent of planned 6MP doses. This discovery, coupled with existing data on the role that TPMT variant alleles also play in 6MP intolerance,4 will help to identify a significant number of children spanning different racial and ethnic groups for individualized 6MP dosing.
Since the first reports that relapse-specific acquired mutations in the gene NT5C2 cause overt resistance to 6MP,5,6 two new studies published in 2015 have helped to expand our knowledge of genes contributing to maintenance therapy resistance. Dr. Xiaotu Ma and colleagues showed that the prevalence of mutations in NT5C2 may be as high as 45 percent in patients with early ALL relapses, and further, that these mutations were often acquired in the founding relapse clone.7 They also observed that patients can acquire multiple mutations in NT5C2, albeit in different subclones, demonstrating the selective pressure on leukemia cells to evade 6MP cytotoxicity. Additionally, Dr. Benshang Li and colleagues reported that another mechanism of thiopurine resistance can occur through the acquisition of relapse-specific mutations in the gene PRPS1.8 PRPS1 is an enzyme involved in the de novo purine biosynthesis pathway, and the acquired mutations were shown to reduce nucleotide feedback inhibition, causing a reduction in the conversion of 6MP into its active metabolite. Mutations were found in 6.7 percent of the B-lineage ALL samples examined and were mutually exclusive of NT5C2 mutations. Taken together, these studies suggest that resistance to 6MP is a commonly observed mechanism of ALL relapse.
While continued advances in the field of pediatric ALL are envisioned with precision medicine initiatives and the development of new targeted agents, the improved utilization of conventional chemotherapy drugs also falls within the spirit of these efforts. The report by Dr. Bhatia and colleagues and the aforementioned studies published in 2015 converge on a central theme of employing different approaches to optimize 6MP delivery during ALL maintenance therapy (Figure). These studies raise important considerations for the development of future prospective ALL trials and suggest that measures to enhance adherence and reduce treatment interruptions will need to be carefully considered. In concert, prospective assessment for TPMT and NUDT15 variants to identify individuals at risk for 6MP intolerance so that their dosing can be refined, and periodic monitoring for the emergence of acquired resistance so that regimens could be modified to avert relapse, may be additional beneficial strategies.
References
Competing Interests
Drs. Raetz and Meyer indicated no relevant conflicts of interest.