In 2015, the vast majority of patients with Hodgkin lymphoma (HL) will be cured of their disease using standard treatments. Despite high rates of long-term disease control, late therapy-related toxicities (most importantly, secondary malignancies and cardiovascular disease) remain a source of significant morbidity and mortality among survivors. In 2015, results from a trio of important risk-adapted trials in early-stage HL were reported on, designed to maintain efficacy while reducing the risk of late effects.
All three trials took the approach of using interim positron emission tomography (PET) scanning to determine how well treatment was working, allowing escalation of therapy for individuals in whom the initial response appeared poor, or reduction of therapy for those with an early metabolic response.
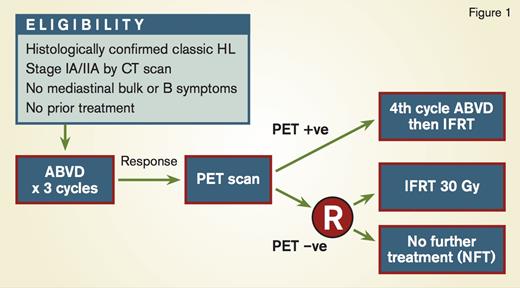
In the RAPID trial from the United Kingdom (Figure 1), patients with newly diagnosed stage IA and IIA nonbulky HL underwent PET imaging after three cycles of ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine).1 PET-positive patients received an additional cycle of ABVD followed by IFRT. Patients who were PET-negative, defined by a Deauville score of 1 or 2 (fluorodeoxyglucose [FDG] uptake was less than the mediastinal blood pool) were randomized to no further therapy versus involved-field radiation therapy (IFRT) to 30Gy. The randomization was designed to exclude a 7 percent or greater difference in three-year progression-free survival (PFS) between the two arms. After two cycles of ABVD, 75 percent of patients were PET-negative, and 420 patients were ultimately randomized. Although the 95 percent confidence interval (CI) for the difference in risk crossed 7 percent (−8.8% to +1.3%), the outcome in both arms was excellent, with a three-year PFS of 94.6 percent in the radiotherapy (RT) arm versus 90.8 percent in the observation arm. There was no difference in overall survival.1
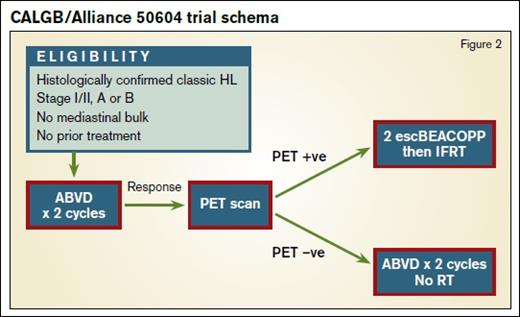
Preliminary results of the U.S. Intergroup study (Figure 2) of risk-adapted therapy in patients with nonbulky stage I and II classical HL were presented at the 57th ASH Annual Meeting in December.2 In this study, Deauville scores of 1 to 3 (FDG uptake less than liver) were considered negative. Patients who were PET-negative after two cycles of ABVD received two more cycles without RT. Individuals who were PET-positive were treated with two cycles of escalated BEACOPP (doxorubicin, bleomycin, vinblastine, and dacarbazine) followed by IFRT to 30Gy. The group treated 164 patients, 91 percent of whom were interim PET-negative. With a median follow-up of two years, the estimated 3-year PFS was 92 percent in the PET-negative arm. In the PET-positive group, the estimated three-year PFS was 66 percent.2 This figure may seem lower than in the PET-positive arm of RAPID, where one further cycle of ABVD followed by 30Gy IFRT yielded 87 percent PFS at five years, but it is important to note that the “PET-positive” group in that trial contained 90 patients with a Deauville score of 3, from a total of 145, which is likely to have increased the PFS substantially.
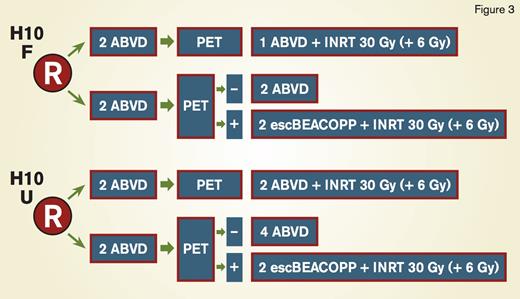
During the 13th International Conference on Malignant Lymphoma in June 2015, results from the H10 study by the European Intergroup (EORTC/LYSA/FIL; Figure 3) were presented.3 Patients with stage I and II disease were randomized between PET-adapted therapy and standard treatment with ABVD for three cycles (favorable patients per the EORTC criteria: age 50 years or older, large mediastinal mass, B symptoms and erythrocyte sedimentation rate [ESR] > 30, ESR > 50 without B symptoms, > 2 nodal sites of involvement) or four cycles (unfavorable patients) plus involved-node RT (INRT) to 30Gy. In the PET-adapted arm, PET-negative patients (Deauville score of 1-3) received a total of four cycles (favorable) or six cycles (unfavorable) of ABVD. Interim PET-positive patients received intensified therapy with escalated BEACOPP for two cycles followed by INRT to 30Gy. In total, 1,950 patients were enrolled, and interim PET was positive in 11 percent and 22 percent for the favorable and unfavorable groups, respectively. With a median follow-up of 4.5 years, among 361 patients who were PET-positive, the 5-year PFS was 91 percent in those on the PET-adapted arm who received BEACOPP, compared with 77 percent in the standard ABVD group. There was also a trend toward improved overall survival in the PET-adapted arm: 96 percent compared with 89 percent (p=0.062).3
These three studies demonstrate the utility of interim PET imaging as a biomarker in patients with early-stage HL. Overall, they suggest that escalation of chemotherapy for PET-positive patients is effective, and they provide evidence to support the idea of omitting RT for those with an early metabolic response. This does not seem to compromise overall survival, though there is a small increase in the risk of recurrence. It is notable that in all three studies the proportion of patients dying from HL was less than 3 percent overall, emphasizing the need to take a holistic view of the data and not focus exclusively on disease control.
Many hematologists/oncologists and patients alike are willing to accept a small increased likelihood of disease recurrence to avoid the risk of secondary cancers and cardiovascular disease associated with RT. To identify patients who may benefit from intensified or novel approaches, interim PET, and possibly molecular predictors, such as the recently described gene expression signature, may be useful tools.4 Additionally, with the availability of novel agents with impressive activity, such as brentuximab vedotin and nivolumab, therapeutic options for high-risk patients will continue to evolve, and trials of these approaches are already underway.
References
Competing Interests
Drs. Johnson and LaCasce indicated no relevant conflicts of interest.