Acquired aplastic anemia (AA) results from autoimmune destruction of hematopoietic progenitor cells, and a significant proportion of these patients develop a myelodysplastic syndrome (MDS) and/or acute myeloid leukemia (AML) as a late complication of their disease.1,2 Recent studies have demonstrated clonal hematopoiesis (CH) in the majority of cases of AA, including pediatric patients and those in whom MDS and AML do not ultimately develop, and these clones are generally detectable early in the course of the disease.1,2 The distribution and types of mutations (frame-shift, nonsense, and splice site changes) identified in AA show significant overlap with those observed in association with MDS and AML but with a much lower allelic burden in the former.1,2,3 Comparison of diagnostic samples with those obtained after six months of immunosuppressive therapy shows no significant difference in the numbers of different mutations present; the levels, however, are significantly increased after treatment.1
The specific genes involved in AA show significant differences from, but also a good deal of overlap with, MDS and AML. Alterations of BCOR, BCORL1, and PIGA are more prevalent in AA, while JAK2, RUNX1, TET2, and TP53 are less so. Mutations of DNMT3A and ASXL1 are noted in both.1,2 Dr. Tetsuichi Yoshizato and colleagues2 suggest discrete mechanisms of clonal selection at play in these processes. In other words, CH in general seems to originate from a small clone present early in the disease course, and its evolution is dependent on the progressive accumulation of additional mutations and subsequent clonal selection.1-4 Thus, CH appears to signify leukemogenesis at a very early stage.
It is generally accepted that stem cells in all tissues, including the bone marrow, tend to accumulate somatic mutations throughout life, most of which are of no clinical significance. Occasionally though, a disease-initiating alteration occurs.4-6 Recent studies suggest that clones with mutations in certain genes seem to have a survival advantage in bone marrow failure.1,2 This seems to occur through either escape from immune-mediated destruction, as with loss-of-function mutations in HLA class I genes, from a relative increase in proliferation, or both.1
An understanding of the genes involved can provide some insight into the mechanism of disease. For instance, the most commonly detected mutation in these series results in a loss of function of DNMT3A, which appears to impede stem cell differentiation and results in accumulation of the abnormal cells within the bone marrow. TET2 mutations (also one of the most frequently identified) facilitate self-renewal and give rise to a competitive growth advantage. It is worth noting that candidate driver mutations, alterations that occur in a subset of genes known to be drivers of hematopoietic neoplasms, are relatively rare among young patients with AA and seem to be enriched in older individuals.3,5
The differences in involved genes are not only of interest for understanding the evolution of disease, but some have also been shown to have prognostic implications. For instance, BCOR, BCORL1, and PIGA mutations generally portend a better response to immunosuppressive therapy and improved overall survival, while abnormalities of ASXL1, CSMD1, DNMT3A, RUNX1, and TP53 tend to herald inferior treatment response and shortened survival. In a similar vein, clones with aberrancies in the former (good prognostic) group of genes tend to remain small or diminish in size over time, whereas clones involving mutations of the latter (poor prognostic) group are more likely to grow in size and progress to MDS and/or AML.
On the clinical front, data presented at the 57th ASH Annual Meeting continue to highlight the promise of the eltrombopag in patients with severe AA. The rationale for using such agents is based on the expression of c-mpl, the receptor for thrombopoietin, on hematopoietic stem and progenitor cells, and the finding that the addition of recombinant thrombopoietin expands the pool of these cells in culture.7 In 2012, Drs. Cynthia Dunbar and Neal Young, and colleagues from the National Institutes of Health (NIH) showed that eltrombopag elicited a 44 percent response rate, including multilineage responses, in patients with severe AA (sAA) refractory to immunosuppression.8 This led to U.S. Food and Drug Administration approval of eltrombopag in this patient population. In the Late-Breaking Abstracts session, Dr. Danielle Townsley, on behalf of colleagues from the same NIH group, presented data that extended these initial results. She presented data from a phase II trial of 88 treatment-naïve sAA patients, where eltrombopag was added to horse antithymocyte globulin (ATG) and cyclosporine (CSA). Eltrombopag was administered starting at day 14 (due to potential concerns for liver toxicity) for six months (cohort 1) or three months (cohort 2), or from day 1 for six months (cohort 3). The combination was well tolerated, and no increased marrow fibrosis was observed. Among the three treatment cohorts, the combination of eltrombopag with ATG + CSA yielded overall response rates at three and six months of 80 percent and 85 percent, respectively; complete response rates were 28 percent and 34 percent at these time points. The addition of eltrombopag yields response rates that are 20 percent to 30 percent higher than historical rates with ATG + CSA alone in sAA (p < 0.001). Clonal cytogenetic evolution occurred in seven of 88 patients, similar to prior rates observed with standard immunosuppression. No baseline factors were predictive of outcome.
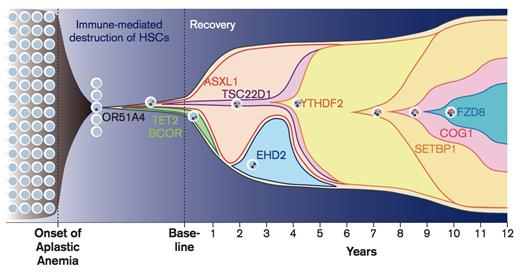
Progression of Disease, Patient NIH075. Chronologic history of clonal evolution from the onset of disease to the last follow-up in Patient NIH075. Each mutated gene is depicted in the representative cells. The vertical axis indicates the absolute volume of the clones. Representative mutations in each clone are also shown. From Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia.N Engl J Med. 2015;373:35-47.
Progression of Disease, Patient NIH075. Chronologic history of clonal evolution from the onset of disease to the last follow-up in Patient NIH075. Each mutated gene is depicted in the representative cells. The vertical axis indicates the absolute volume of the clones. Representative mutations in each clone are also shown. From Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia.N Engl J Med. 2015;373:35-47.
2015 was a breakthrough year for AA. The marriage of clinical breakthroughs with a more sophisticated understanding of the mutational landscape of AA is certain to catalyze robust translational research opportunities moving forward. Additional investigation is needed to correlate both baseline mutation status and temporal changes in clonal architecture (Figure) with disease natural history and response to immunosuppression and hematopoietic stem cell transplantation.
References
Competing Interests
Drs. George and Harrison indicated no relevant conflicts of interest.