Macrophages are key regulators of both innate and adaptive immunity. Classically, macrophages are thought of as scavengers, phagocytizing dead cells and debris, but they can also act as sentries, on the lookout for foreign invaders and ready to call in reinforcements from the adaptive immune system when needed.
A key regulator of the ability of macrophages to phagocytose a cell is through the expression of CD47, often dubbed a “don’t-eat-me” signal. CD47 partially acts as a marker of self, preventing phagocytosis of circulating red blood cells in mice1 or of cells during transplantation.2 Work from Dr. Irv Weissman’s group demonstrated that circulating hematopoietic stem cells express CD47 to prevent macrophage clearance and that malignancies up-regulate the don’t-eat-me signal as an “invisible cloak” to evade innate immune clearance.3,4 This has prompted therapeutic strategies in both blood and solid organ malignancies to target CD47 with antibodies, or other blocking agents, to remove the don’t-eat-me signal and allow for tumor clearance.
The pre-clinical animal models used to develop anti-CD47 therapies largely relied on xenotransplantation into immunocompromised mice and showed that macrophages are the key cellular player in their therapeutic effect. However, since these models lack adaptive immunity, it has been unclear what role antigen presentation and activation of T cells play in anti-CD47 therapy.
A new report in Nature Medicine by Dr. Xiaojuan Liu and colleagues has employed two separate immune-competent mouse models to explore the role of adaptive immunity in anti-CD47 treatment. When wild-type BALB/c mice were inoculated with a syngeneic B-cell lymphoma cell line, anti-CD47 treatment resulted in clearance of the tumor and prolonged survival. Using a solid tumor in C57Bl/6 mice produced a similar result. However, when a similar experiment was performed in nude BALB/c mice, which are athymic and lack functional T cells, the same anti-CD47 regimen failed to alter tumor growth, suggesting that T-cell function was a key requirement of therapeutic efficacy in immune-competent settings. The authors further demonstrated a T-cell requirement by co-treating tumor-bearing mice with anti-CD47 and either anti-CD4 or anti-CD8 antibodies, which revealed that depletion of CD8+ T cells abrogated the therapeutic response to anti-CD47 therapy.
While CD8+ T cells were required for effective antitumor responses, the role of macrophage antigen presentation was still unknown. In in vitro assays, the authors showed that dendritic cells, rather than macrophages, were the primary activators of T cells. Using a mouse model, in which CD11c+ dendritic cells could be specifically deleted in vivo, they then showed that depletion of dendritic cells resulted in a lack of effectiveness of anti-CD47 therapy, while depletion of tumor-associated macrophages had no impact on the antitumor response.
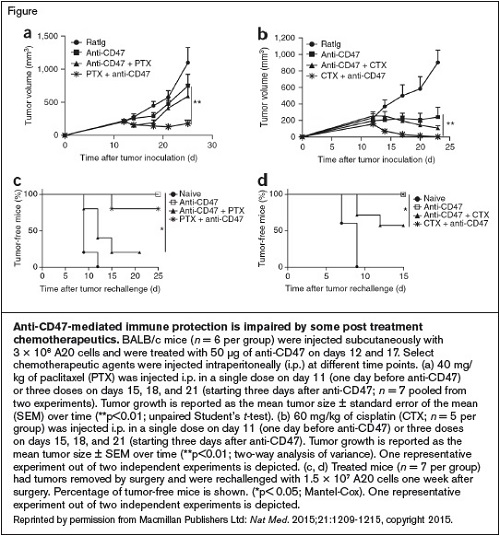
Anti-CD47-Mediated Immune Protection is Impaired by Some Post Treatment Chemotherapeutics. BALB/c mice (n = 6 per group) were injected subcutaneously with 3 × 106 A20 cells and were treated with 50 μg of anti-CD47 on days 12 and 17. Select chemotherapeutic agents were injected intraperitoneally (i.p.) at different time points. (a) 40 mg/ kg of paclitaxel (PTX) was injected i.p. in a single dose on day 11 (one day before anti-CD47) or three doses on days 15, 18, and 21 (starting three days after anti-CD47; n = 7 pooled from two experiments). Tumor growth is reported as the mean tumor size ± standard error of the mean (SEM) over time (**p<0.01; unpaired Student’s t-test). (b) 60 mg/kg of cisplatin (CTX; n = 5 per group) was injected i.p. in a single dose on day 11 (one day before anti-CD47) or three doses on days 15, 18, and 21 (starting three days after anti-CD47). Tumor growth is reported as the mean tumor size ± SEM over time (**p<0.01; two-way analysis of variance). One representative experiment out of two independent experiments is depicted. (c, d) Treated mice (n = 7 per group) had tumors removed by surgery and were rechallenged with 1.5 × 107 A20 cells one week after surgery. Percentage of tumor-free mice is shown. (*p< 0.05; Mantel-Cox). One representative experiment out of two independent experiments is depicted.Reprinted by permission from Macmillan Publishers Ltd: Nat Med. 2015;21:1209-1215, copyright 2015.
Anti-CD47-Mediated Immune Protection is Impaired by Some Post Treatment Chemotherapeutics. BALB/c mice (n = 6 per group) were injected subcutaneously with 3 × 106 A20 cells and were treated with 50 μg of anti-CD47 on days 12 and 17. Select chemotherapeutic agents were injected intraperitoneally (i.p.) at different time points. (a) 40 mg/ kg of paclitaxel (PTX) was injected i.p. in a single dose on day 11 (one day before anti-CD47) or three doses on days 15, 18, and 21 (starting three days after anti-CD47; n = 7 pooled from two experiments). Tumor growth is reported as the mean tumor size ± standard error of the mean (SEM) over time (**p<0.01; unpaired Student’s t-test). (b) 60 mg/kg of cisplatin (CTX; n = 5 per group) was injected i.p. in a single dose on day 11 (one day before anti-CD47) or three doses on days 15, 18, and 21 (starting three days after anti-CD47). Tumor growth is reported as the mean tumor size ± SEM over time (**p<0.01; two-way analysis of variance). One representative experiment out of two independent experiments is depicted. (c, d) Treated mice (n = 7 per group) had tumors removed by surgery and were rechallenged with 1.5 × 107 A20 cells one week after surgery. Percentage of tumor-free mice is shown. (*p< 0.05; Mantel-Cox). One representative experiment out of two independent experiments is depicted.Reprinted by permission from Macmillan Publishers Ltd: Nat Med. 2015;21:1209-1215, copyright 2015.
Given the important role of adaptive immunity in the antitumor response, the authors explored various scenarios in addition to the monotherapy, such as coupling anti-CD47 treatment with either cyclophosphamide or paclitaxel. These experiments demonstrated that chemotherapy should be given prior to anti-CD47 rather than after (Figure) to allow for synergistic effects and maintenance of the responsive CD8+ T cells for long-term immune surveillance.
In Brief
This study elegantly demonstrates in immune-competent mouse models that the therapeutic effects of anti-CD47 therapy are a result of both innate and adaptive immune responses. Blocking CD47 does much more than ring the dinner bell for macrophages to devour tumor cells. Rather it allows for effective antigen presentation by dendritic cells, priming CD8+ T-cell responses against the tumor. T-cell responses resulting from anti-CD47 treatment are consistent with a prior report,5 but in contrast to the earlier study, the authors claim that dendritic cells, rather than macrophages, drive the antigen presentation. They also attribute some of the discrepancies to in vitro culture conditions with or without serum. Regardless of the cell responsible for T-cell priming, the report by Dr. Liu and colleagues highlights the importance of immune responses in anti-CD47 therapies and suggests that unique timing and sequences in conjunction with other therapeutics should be explored to optimize efficacy.
References
Competing Interests
Dr. Hoggatt indicated no relevant conflicts of interest.