The overall survival for patients with follicular lymphoma has improved dramatically throughout the past several decades, largely due to the introduction of rituximab. A subset of patients, however, will experience early relapse after up-front therapy, which recent studies suggest is strongly associated with poor outcomes. The Follicular Lymphoma International Prognostic Index (FLIPI) was developed prior to the routine use of rituximab and identified five risk factors: age, stage, lactate dehydrogenase, hemoglobin, and number of involved lymph nodes sites. Although it is a useful clinical tool in predicting disease behavior, the FLIPI does not reliably identify these highest-risk patients.
In a recent large-scale analysis of patients receiving first-line chemotherapy, Dr. Alessandro Pastore and colleagues performed DNA deep sequencing of 151 follicular lymphoma biopsy specimens in patients uniformly treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone on a phase III clinical trial. After incorporating baseline clinical factors, they developed a risk model for failure-free survival. The model was then validated in a second cohort of 107 patients uniformly treated with rituximab plus cyclophosphamide, vincristine, and prednisone.
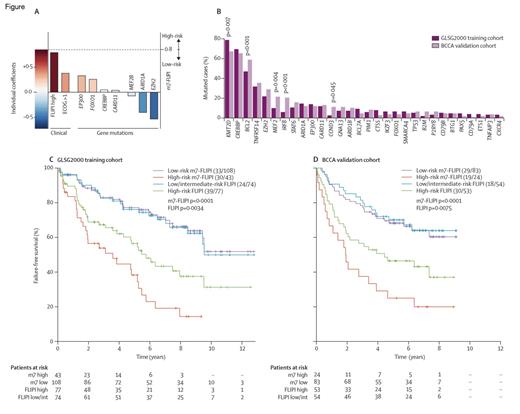
The Clinicogenetic Risk Model m7-FLIPI. Reprinted from Lancet Oncology, Vol 16/edition 9, Pastore A et al, Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry, 1111-1122, Copyright 2015, with permission from Elsevier.
The Clinicogenetic Risk Model m7-FLIPI. Reprinted from Lancet Oncology, Vol 16/edition 9, Pastore A et al, Integration of gene mutations in risk prognostication for patients receiving first-line immunochemotherapy for follicular lymphoma: a retrospective analysis of a prospective clinical trial and validation in a population-based registry, 1111-1122, Copyright 2015, with permission from Elsevier.
The median number of gene mutations identified in the training set was four, and nine genes were mutated in more than 10 percent of specimens. 97 percent and 46 percent of lymphomas were found to harbor mutations in epigenetic modifiers and transcription factors, respectively. A clinicogenetic model, termed M7FLIPI, consisting of the FLIPI risk factors, Eastern Cooperative Oncology Group performance status, and mutations in seven genes (i.e., EZH2, ARID1A, EP300, FOXO1, MEF2B, CREBBP, and CARD11) was constructed and was more closely associated with outcome compared to the clinical or genetic predictors alone. Two risk groups were identified: a high risk group (28% of patients) with five-year failure-free survival (FFS) of 38 percent, and a low-risk group (72% of patients) with a five-year FFS of 77 percent. Analysis of the validation cohort revealed similar results. In addition, the M7FLIPI correlated with five-year overall survival of 65 percent and 90 percent, respectively, in high- and low-risk patients. Interestingly, approximately half of patients classified as high risk according to FLIPI were categorized as low risk using M7FLIPI, predominately driven by mutations in EZH2. Mutations in MEF2B and ARID1A were also associated with improved outcomes. The high-risk group, in contrast, was enriched with mutations in EP300 and CREBBP.
In Brief
The M7FLIPI is the first prognostic score in lymphoma to incorporate both genetic and clinical factors, resulting in the identification of a high-risk group in patients treated with standard chemoimmunotherapy. Moving forward, the M7FLIPI will be of great utility in both the design of clinical trials and the management of patients. With a disease characterized by a median overall survival of more than 15 years, patients with favorable-risk disease should receive lower-intensity approaches. Efforts should focus on novel drugs and combinations, possibly including consolidation or maintenance strategies for the minority of patients who have high-risk disease. For both groups, tailoring therapy based on the mutational profile may facilitate the omission of standard chemotherapy with the hope of improved outcomes with less toxicity.
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest.