The Question
What is your approach to the diagnosis and treatment of patients with low-risk myelodysplastic syndrome (MDS) related to immune pathophysiology?
Case
A 53-year-old man was found to have pancytopenia during follow-up for hyperuricemia. The laboratory findings were as follows: WBC, 2.44×109/L with 26 percent neutrophils, 1 percent eosinophils, 8 percent monocytes, and 65 percent lymphocytes; RBC, 1.49×1012/L; hemoglobin, 5.7 g/dL; MCV, 112.8 fl; platelets, 22×109/L; reticulocytes, 45×109/L; LDH=195 IU/L. The patient’s bone marrow (BM) was normocellular with 2 percent blasts and showed some signs of dysplasia in erythroid precursors and granulocytes without increased ring sideroblasts. The megakaryocyte count was decreased. The karyotype of the BM cells was 46, XY in 20 dividing cells. The patient was therefore diagnosed as having refractory cytopenia with multilineage dysplasia (RCMD) with an International Prognostic Scoring System (IPSS) risk group of intermediate-1 (IPSS-revised risk category: intermediate). His physician suggested that he receive red blood cell transfusions and possibly hematopoietic stem cell transplantation or azacitidine therapy if the pancytopenia progressed further.
My Response
Distinguishing Cases of Immune-Mediated BM failure
Diagnosing MDS is often challenging in patients with pancytopenia or bicytopenia who do not show definitive poor prognostic markers, such as karyotypic abnormalities or increased blasts or ring sideroblasts in the BM, as in the present case. The differential diagnosis of such cases of gray zone BM failure includes refractory cytopenia with unilineage dysplasia, RCMD, idiopathic cytopenia(s) of undetermined significance, and moderate aplastic anemia (AA).1 It is generally difficult to make an exact diagnosis based on the established diagnostic criteria because these disease entities overlap, and each diagnosis relies on a subjective judgement of the cell morphology by physicians and/or pathologists.2-4 Giving a disease name to such an equivocal condition has virtually little value. Instead, it is clinically relevant to distinguish benign subsets of BM failure that are likely to respond to immunosuppressive therapy (IST) from cases of non–immune-mediated BM failure, including those associated with pre-leukemic features and inherited BM failure syndromes.
Difficulties in Evaluating BM Cellularity in Patients with BM Failure
BM aspiration and trephine biopsies are essential for obtaining the differential diagnosis of BM failure syndromes, and the detection of a hypocellular BM is a prerequisite for diagnosing AA.5 However, assessing BM cellularity in patients with BM failure is often difficult, particularly when the cytopenia(s) are not severe. Even when the BM of one bone site is grossly replaced with fat tissue as a result of the immune-mediated destruction of hematopoietic stem cells (HSCs), some hematopoietic nests remain in other bone sites and may show hypercellularity due to increased BM activity that compensates for the decreased hematopoiesis.6 BM aspiration or biopsies of these hot spots can sometimes produce erroneous results. When pathological reports of BM examinations show hyper- or normal cellularity, the physician may not generally consider the differential diagnosis of AA. Hematologists should be careful with respect to cellularity when interpreting the results of BM examinations, considering that BM cellularity cannot be accurately determined based on BM biopsies of limited sites in the iliac bone. Magnetic resonance imaging (MRI) of the thoracolumbar spine can be used to supplement an assessment of cellularity with a BM biopsy. However, MRI often produces equivocal results in elderly patients owing to age-related fatty changes in the BM. Therefore, hematologists should keep in mind that BM hypercellularity does not necessarily preclude a diagnosis of immune-mediated BM failure.
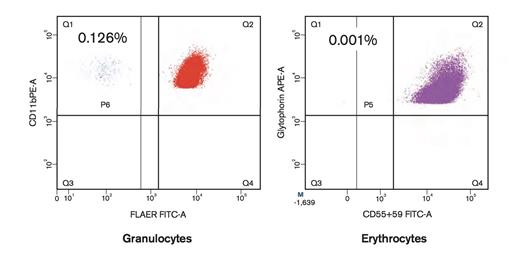
Detection of PNH-Type Cells Using High Sensitivity Flow Cytometry. Detection of paroxysmal nocturnal hemoglobinuria (PNH) -type cells using high-sensitivity flow cytometry. The patient possessed increased PNH-type cells only in granulocytes.
Detection of PNH-Type Cells Using High Sensitivity Flow Cytometry. Detection of paroxysmal nocturnal hemoglobinuria (PNH) -type cells using high-sensitivity flow cytometry. The patient possessed increased PNH-type cells only in granulocytes.
Laboratory Markers Representing Immune Pathophysiology of BM Failure
The presence of predominant thrombocytopenia is the most fundamental feature of immune-mediated BM failure that should prompt further examinations of other laboratory markers. An increased percentage of paroxysmal nocturnal hemoglobinuria (PNH)–type cells, which can be detected using high-sensitivity flow cytometry (FCM), represents a reliable marker of immune pathophysiology.7,8 The usefulness of detecting increased PNH-type cells for predicting the response to immunosuppressive therapy (IST) in AA patients was confirmed in recent prospective studies utilizing high-sensitivity FCM-based methods that can precisely measure 0.01 percent or more PNH-type cells.9,10 Figure 1 shows the results of FCM in the current case, which revealed 0.126 percent of granulocytes and 0.001 percent of erythrocytes to be PNH-type cells. Such results can be obtained on the same day as the patient’s visit, and if those results are positive, physicians have the option of commencing IST shortly thereafter. The presence of HLA-DRB1*15:01 has been reported to predict a good response to IST in patients with MDS.11 However, in our previous study using a multivariate analysis of several factors, including the presence or absence of DRB1*15:01, only the detection of PNH-type cells was associated with a good response to IST in patients with AA.12 Therefore, DRB1 typing to clarify the immune pathophysiology of BM failure is unnecessary if high-sensitivity FCM is available and detects PNH-type cells.
Another solid marker of immune pathophysiology in patients with BM failure is the presence of HLA-A allele–lacking leukocytes (HLA-LLs) derived from HSCs that develop copy number–neutral loss of heterozygosity of the HLA haplotype owing to uniparental disomy of the short arm of chromosome 6 (6pUPD).13 Detecting 6pUPD(+) leukocytes requires a single nucleotide polymorphism array analysis, which takes several weeks. On the other hand, HLA-LLs can be easily detected using monoclonal antibodies specific to HLA-A alleles with FCM if the patient is heterozygous for the HLA-A allele.
Another useful examination that does not require advanced techniques is measurement of the plasma thrombopoietin (TPO) level. Patients with MDS who have a TPO level ≥320 pg/mL (TPOhigh patients) exhibit a high progression-free survival rate and good response to IST, similar to cases of AA.14 Virtually all patients having increased PNH-type cells or HLA-LLs fall into the TPOhigh group. Accordingly, if data for the TPO level are available, physicians may not need to examine the peripheral blood in patients with BM failure to determine the presence of other markers. The recent National Comprehensive Cancer Network guidelines recommend using hypomethylating agents for the treatment of MDS associated with thrombocytopenia; however, this option may be hazardous to TPOhigh patients, as BM failure in these cases is not based on the presence of abnormal stem cells with pre-leukemic features.15
Nakao 9/21/15
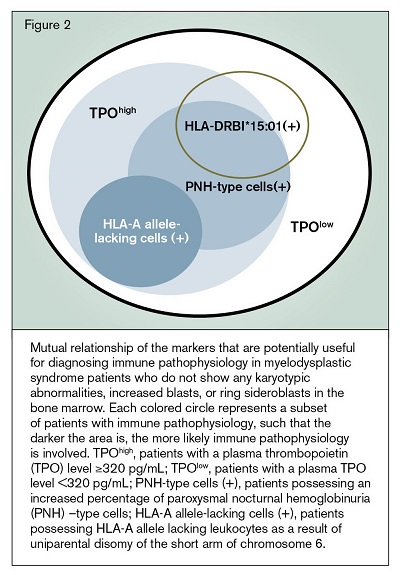
Figure 2 illustrates the mutual relationship of the markers that are potentially useful for diagnosing immune pathophysiology in patients who do not show any karyotypic abnormalities, increased blasts or ring sideroblasts in the BM. The precise role of these markers in the management of gray zone BM failure must be evaluated in a large prospective study because the predictive values of HLA-LLs and plasma TPO levels have only been assessed in Japanese patients with BM failure. When deciding on treatment, physicians also need to take patient age and disease duration into account, both of which are known to affect the response to IST.11
Patient Follow-up
Based on the positive results for PNH-type granulocytes, we treated the patient with cyclosporine at a dose of 4 mg/kg/day. The pancytopenia gradually improved in response to this therapy, and the blood cell count seven months after the initiation of treatment was as follows: WBC, 3.0×109/L with 44 percent neutrophils; RBC, 2.79×1012/L; hemoglobin, 9.4 g/dL; MCV, 101.4 fl; platelets, 57×109/L; reticulocytes, 75×109/L. He continues to receive cyclosporine without any signs of significant toxicities.
References
Competing Interests
Dr. Nakao receives research funds and honoraria from Alexion.