In contrast to high cure rates for newly diagnosed pediatric acute lymphoblastic leukemia (ALL), relapsed disease remains difficult to treat effectively and is a leading cause of childhood cancer deaths.1 One of the challenges in the clinical management of relapsed ALL is that it often occurs unpredictably in the absence of traditional high-risk prognostic factors. Recent genomic studies have investigated the evolutionary trajectory of drug-resistant clones from diagnosis to relapse and have provided greater insight into the biological mechanisms of disease recurrence. Most studies, however, have analyzed the genetic lesions in the clones that survive frontline therapy (the rising clones), but not in those successfully eradicated by treatment (the falling clones).
In this study, Dr. Xiaotu Ma and colleagues analyzed diagnosis, remission, and relapse samples from 20 children with B-progenitor ALL (B-ALL), studied as a part of a collaborative effort from the Children’s Oncology Group (COG), the National Cancer Institute Therapeutically Applicable Research to Generate Effective Treatments (TARGET) initiative, and the St. Jude–Washington University Pediatric Cancer Genomic Project. Their goal was to address key questions in clonal evolution from diagnosis to relapse using deep genome-wide sequencing of matched samples from individual patients. Somatic sequence mutations, structural variations (SVs), and DNA copy-number alterations were analyzed. Using this methodology, 99 percent of the mutations with a mutant allele fraction (MAF) of at least 0.01 could be detected.
All cases analyzed were from children with early bone marrow relapses occurring within 36 months (median, 19.2 months) from diagnosis—historically the most challenging group to treat. Seventy-two percent of the mutations had MAF of <0.3, suggesting they were subclonal. In characterizing the somatic mutation profile of patients, investigators demonstrated that the majority of the coding sequence mutations present at diagnosis (74%) persisted at relapse, supporting the notion of a common preleukemic clone. In 16 cases, the number of coding somatic sequence mutations at relapse was almost two-fold higher than at diagnosis (median 31 vs. 18, p=0.004). Certain mutations were relapse-specific, and notably, mutations in NT5C2, a gene encoding a 5'-nucleotidase involved in purine metabolism and conferring resistance to thiopurines,2,3 were observed exclusively at relapse in 45 percent of the cases.
The authors also identified six key pathways that were most commonly implicated at diagnosis and/or relapse. These pathways were: Ras signaling (65%), JAK-STAT signaling (25%), transcriptional regulation of lymphoid development (85%), nucleoside metabolism (45%), epigenetic modification (65%), and cell cycle regulation (60%). Interestingly, there were cases in which several subclonal mutations in a common pathway at diagnosis converged to a single predominant mutation within the same pathway at relapse.
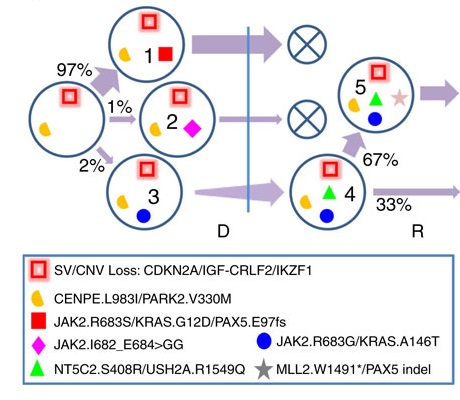
Clonal Lineages at Diagnosis (D) and Relapse (R). Each clone is identified with a number. Mutation clusters present in each clone are marked by distinct shapes and colors. Key mutations as well as copy-number variation and structural variations in each cluster are labelled in the legend. D and R are demarcated by a vertical blue bar. Clonal population size is labelled as percentage of tumor content. The thickness of an arrow from a progenitor clone to its descendant is proportional to population change. ”Falling” clones that did not survive therapy are marked by an X.
Clonal Lineages at Diagnosis (D) and Relapse (R). Each clone is identified with a number. Mutation clusters present in each clone are marked by distinct shapes and colors. Key mutations as well as copy-number variation and structural variations in each cluster are labelled in the legend. D and R are demarcated by a vertical blue bar. Clonal population size is labelled as percentage of tumor content. The thickness of an arrow from a progenitor clone to its descendant is proportional to population change. ”Falling” clones that did not survive therapy are marked by an X.
The authors demonstrated dynamic changes in the individual cases to illustrate how clonal evolution led to the turnover of the predominant mutations at diagnosis and the acquisition of relapse-specific mutations. Notably, in 15 (75%) of 20 cases, relapsed tumors were descendants of minor subclones at diagnosis, suggesting that in most cases, the predominant clones at diagnosis were successfully eradicated by treatment. This is depicted in the Figure, where relapse emerged from a minor subclone with a population frequency of 2 percent at diagnosis.
The authors also evaluated how the mutation burden, profile, and frequency changed during the evolution from diagnosis to relapse. Clonal diversity was comparable at diagnosis and relapse, with the median number of three (range, one 1-5) subclones identified at both time points. There were also no differences in the number of mutations in the rising clones (median, 11; range, 2-32) compared with that in the falling clones (median, 11; range, 1-44; p=0.7), indicating that clonal survival is not dependent on mutation burden. A further key observation was that mutant alleles shared between diagnosis and relapse were detectable in the remission samples at the end of induction in the majority of cases, suggesting that there may be clinical utility to assaying for specific mutations that could be a harbinger of relapse during the course of front-line therapy.
In Brief
This study by Dr. Ma and colleagues provides further insight into the genetic basis of treatment failure in childhood ALL, detailing the trajectory of clonal emergence from diagnosis to relapse and demonstrating that the majority of relapses evolve from a minor subclone present at diagnosis, which is detectable at the end of induction therapy. Given the challenges in successfully treating relapse once it occurs, these findings offer promise for earlier detection of genetic lesions that could be a harbinger of relapse, so alternative therapies might be instituted prior to overt disease recurrence.
Figure. Clonal lineages at diagnosis (D) and relapse (R). Each clone is identified with a number. Mutation clusters present in each clone are marked by distinct shapes in colors that match those in a. Key mutations as well as copy-number variation and structural variations in each cluster are labelled in the legend. D and R are demarcated by a vertical blue bar. Clonal population size is labelled as percentage of tumor content. The thickness of an arrow from a progenitor clone to its descendant is proportional to population change. ”Falling” clones that did not survive therapy are marked by an X.
Reprinted from Nature, Vol 6, Ma X, Edmonson M, Yergeau D, et al., Rise and fall of subclones from diagnosis to relapse in pediatric B-acute lymphoblastic leukaemia, Article No. 6604, Copyright 2015.
References
Competing Interests
Dr. Raetz indicated no relevant conflicts of interest.