Study Title:
A Phase 1-2 Multi-Center Study Evaluating KTE-C19 in Subjects With Refractory Aggressive Non-Hodgkin Lymphoma (NHL)
ClinicalTrials.gov Identifier:
Sponsor:
Kite Pharma, Inc.
Participating Centers:
Four centers in the United States
Accrual:
124 participants
Study Design:
Kite Pharma is conducting a multicenter phase I/II study of KTE-CD19 in patients with refractory diffuse large B-cell lymphoma (DLBCL), primary mediastinal large B-cell lymphoma, and transformed follicular lymphoma. The primary end points of the phase I and phase II portions of the study are safety and objective response rate, respectively. Collected via leukapheresis, peripheral blood mononuclear cells are cultured with an anti-CD3 antibody and IL-2 and then transduced with a third-generation chimeric antigen receptor (CAR) T-cell construct containing an anti-CD19 scVf conjugated to a CD28 costimulatory molecule and CD3 zeta chain. Patients then receive fludarabine and cyclophosphamide followed by CAR T-cells at a target dose of 2×106 cells/kg.
Rationale:
Although the majority of patients with DLBCL are cured with upfront chemoimmunotherapy, approximately 35 percent of patients will experience relapsed or have primary refractory disease. Fewer than half of these patients will be candidates for high-dose chemotherapy with autologous stem cell rescue as a result of failure to respond to second line therapy or due to age and/or medical co-morbidities. Additionally, disease recurrence after transplant is common. Despite advances in understanding the biology of DLBCL, including the identification of high-risk molecular features such as rearrangements and protein overexpression of MYC and BCL-2, effective therapies for relapsed DLBCL are desperately needed. Harnessing the immune system using CAR T-cells has emerged as a promising, novel approach in this setting.
CD19 is expressed on nearly all normal and malignant B-cells with the exception of plasma cells, and is not found on hematopoietic stem cells or other cell types in the body. Anti-CD19 CAR T cells are autologous T cells, collected by peripheral blood pheresis, engineered to express a single chain variable fragment (scVf), derived from a monoclonal anti-CD19 antibody, and linked to costimulatory molecules.
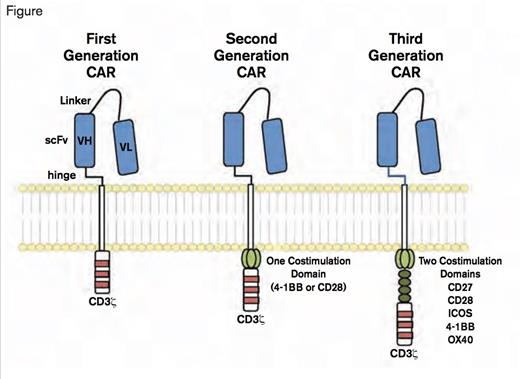
Chimeric Antigen Receptors. CARs target surface antigens in an MHC-independent fashion and consist of an ectodomain, hinge domain, transmembrane domain, and endodomain. The initial trials tested first-generation CARs that have a single cytoplasmic domain. Current trials are testing second- and third-generation CARs that have combinations of signaling domains. (Blood. 2014;123:2625-2635.)
Chimeric Antigen Receptors. CARs target surface antigens in an MHC-independent fashion and consist of an ectodomain, hinge domain, transmembrane domain, and endodomain. The initial trials tested first-generation CARs that have a single cytoplasmic domain. Current trials are testing second- and third-generation CARs that have combinations of signaling domains. (Blood. 2014;123:2625-2635.)
A recently published study of anti-CD19 CAR T-cells from the NCI demonstrated impressive activity in a cohort of 15 patients with heavily pretreated B-cell lymphoma, nine of whom had DLBCL, including four with primary mediastinal large B-cell lymphoma (Figure; Kochenderfer JN, et al. J Clin Oncol. 2015;33:540-549; Maus MV, et al. Blood. 2014;123:2625-2635). Their third generation CAR T-cell construct contained an anti-CD19 scVf conjugated to a CD28 costimulatory molecule and CD3 zeta chain designed to enhance T-cell activation. After undergoing pheresis, patients were pre-treated with cyclophosphamide and fludarabine to prevent destruction of infused anti-CD19 CAR T cells. Of the seven evaluable patients with DLBCL (one was lost to follow-up and a second died approximately two weeks after CAR T-cell infusion of a presumed cardiac arrhythmia), four achieved a complete response, two had a partial response, and one experienced stable disease. Adverse events consisted primarily of acute reactions following cell infusion, including fever and hypotension, as well as reversible neurologic toxicity ranging from delirium to focal neurologic deficits and myoclonus in the setting of elevated serum levels of interferon-γ and interleukin-6.
Comment:
CAR T-cell therapy represents a promising novel approach in patients with refractory aggressive B-cell lymphoma. Cytotoxic chemotherapy, consisting of combinations of drugs with diverse mechanisms of actions, even at high doses, has yielded disappointing results in relapsed DLBCL, particularly since the incorporation of rituximab in up-front therapeutic regimens. This appears to be particularly true in double hit and double protein-expressing lymphomas, which are especially resistant to chemotherapy and for which no effective salvage therapies have been developed. Employing an immunologic approach, which may be active across multiple underlying molecular drivers of disease, is highly appealing. If this approach proves effective in the refractory setting, it could then be studied as consolidation in previously untreated high-risk patients.
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest.