The ASH Meeting on Lymphoma Biology was held August 10-13, 2014, to establish a collaborative forum for the community of lymphoma researchers, enable them to share data, and ultimately, advance the understanding of lymphoma pathogenesis and expedite new therapies. With assistance from ASH, a steering committee of lymphoma experts both organized the meeting and developed a "Roadmap for Discovery and Translation in Lymphoma," which was recently published in Blood. In this companion article, we will summarize the current landscape of challenges, as well as advances, that provide the backdrop for the roadmap and the priorities at its foundation.
There are more than 80 unique subtypes of mature lymphoid malignancy, including non-Hodgkin lymphomas (NHLs), Hodgkin lymphoma (HL), and chronic lymphocytic leukemia (CLL). Many of these subtypes are rare, with 5,000 or fewer cases occurring in the United States annually. The association between some subtypes and pre-existing conditions, including immunodeficiencies, autoimmune disorders, and infections (e.g., HIV, hepatitis C virus, Epstein-Barr virus [EBV]), further complicates both categorization and treatment.1
Despite this diversity, therapeutic advances in lymphoma have been critically important in cancer medicine since the administration of nitrogen mustard to a patient with NHL in 1942. Treatment of endemic Burkitt lymphoma in the 1950s was one of the first demonstrations that chemotherapy could cure cancer. Combination chemotherapy with MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone), developed by investigators at the National Cancer Institute in the late 1960s, cured more than one-half of patients with HL. The addition of rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) for diffuse large B-cell lymphoma (DLBCL) established the paradigm for increasing cure rates by combining chemotherapy with immunotherapy. EBV-directed T cells for post-transplant action lymphoproliferative disorders was the first curative cellular therapy that specifically targeted malignant cells. Antibiotic treatment of early-stage mucosa–associated lymphoid tumors (MALT) of the stomach was the first demonstration that modulation of the gut microbiome can eradicate a neoplastic process. Finally, a deeper understanding of lymphoma pathogenesis has guided the development of targeted therapies such as ibrutinib and idelalisib for patients with CLL, mantle cell lymphoma, and follicular lymphoma.
Based on these advances, approximately 90 percent of patients with HL and more than 60 percent of patients with DLBCL in the United States will be cured of their disease. Among patients with low-grade lymphomas (e.g., follicular lymphoma, marginal zone lymphomas, small lymphocytic lymphoma/CLL), median survival for patients diagnosed in 2015 is likely to significantly exceed 10 years from diagnosis. As a result, the ultimate goal of providing tolerable and curative therapy to the overwhelming majority of patients with lymphoma seems to be realistic within the foreseeable future, but only if significant challenges can be overcome. These include:
1) Empiric chemotherapy regimens such as CHOP and ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine) remain the backbone of initial therapy for the majority of patients. Curative salvage therapies largely involve high-dose chemotherapy with or without stem cell transplantation. These approaches are associated with significant toxicity that precludes their use in patients with significant comorbidities and in some developing countries where adequate supportive care is not available. 2) The outcomes for some patients, including many with peripheral T-cell lymphomas (PTCL) or with "double-hit lymphomas" that harbor rearrangements of both MYC and BCL2, remain dismal. 3) The investigation of rare lymphoma subtypes is limited by inadequate numbers of representative cell lines and in vivo models, and by the incomplete characterization of genetic, epigenetic, transcriptional, proteomic, and metabolomic landscapes across each subtype. Notably, DLBCL was the only lymphoma subtype included in The Cancer Genome Atlas (TCGA) project, and DLBCL sequencing was prematurely discontinued because of a lack of adequate sample procurement. 4) Insufficient collaboration exists across centers, which reduces both the cross-fertilization of expertise and the availability of specimens.
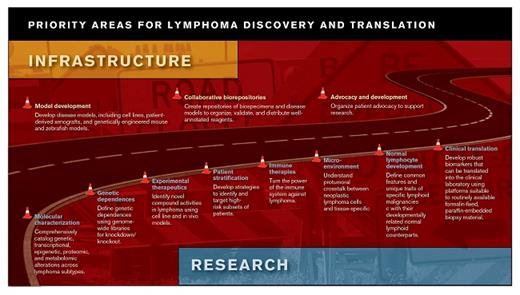
Each of these challenges limits the expedient development of novel therapies for patients with lymphoma. Yet, National Institutes of Health (NIH) funding for research and cooperative trial groups has been significantly curtailed. To address this disconnect between available funding and the extraordinary opportunity for advances in lymphoma treatment, experts within the lymphoma biology community gathered to establish a set of priorities for future research and infrastructure efforts. The roadmap developed by the steering committee of the ASH Meeting on Lymphoma Biology includes a list of such priorities for future research and infrastructure that are meant to facilitate the next generation of major advances in lymphoma treatment. These priorities are intended to drive funding allocations at the NIH and other governmental agencies, to support advocacy, and to direct effort and resources toward the areas of greatest potential benefit.
The priorities outlined in the roadmap are generally ambitious but are intended to be realistic targets for a five-year funding cycle. For example, the roadmap calls for the whole genome sequencing, RNA sequencing, and phosphoproteome analysis of ≥ 500 primary specimens (with paired germline sequencing) from each common lymphoma subtype and ≥ 50 from each less common subtype. While this number of samples will not allow for a comprehensive catalog of the lymphoma landscape, it will capture a very large fraction of the heterogeneity across lymphomas. Similarly, genome-wide screens across all relevant lymphoma cell lines will help define a broad range of dependencies and synthetic lethal interactions with current therapies that can be exploited with novel agents. Recent studies demonstrating the remarkable activity of some immune therapies in patients with mature lymphoid malignancies2-4 indicate that efforts to understand and modulate both the immune response and lymphoma microenvironment should also be high priorities.
The establishment of cell line and in vivo models for subtypes that currently lack models (e.g., many low-grade lymphomas and subtypes of PTCL) is a high priority. As techniques advance for generating these reagents, the number of representative models should increase with our understanding of the genetic and epigenetic underpinnings of lymphoma. Optimally, the models and associated genomic characterization would be available from repositories that foster the greatest possible distribution to both academic and industry investigators. Models for this kind of distribution (e.g., ATCC, JAX, WiCell) already exist and, with appropriate safeguards for patient confidentiality and informed consent, could be extended to large numbers of lymphoma cell lines, primary cell cultures, patient-derived xenografts, genetically engineered mouse models, and spontaneous tumor models.
In parallel with strengthening multicenter collaboration to expand our understanding of lymphoma biology, similar efforts to improve infrastructure are required with respect to therapeutics. As an increasing number of biologically defined subsets of lymphoma are elucidated, identifying patients appropriate for rationally designed trials will become more challenging. Current therapies are either curative or able to induce long remissions across a range of lymphomas. Thus, the identification of patients who are likely to have poor outcomes with current therapies is a high priority. This may involve the development of prognostic indices that incorporate biologic determinants of risk with clinical factors. Validated indices can then be used to stratify patients in clinical trials. Novel study designs and biomarker-driven endpoints are also necessary, as is the participation of many sites to accrue adequate numbers of patients. Streamlining the current regulatory and administrative processes to conduct clinical trials is critical, as the translation from the laboratory to the clinic remains unnecessarily slow. Additionally, clinical trials should incorporate re-biopsies as a routine standard whenever possible. This is essential for supporting the identification of response biomarkers, the elucidation of mechanisms of therapeutic resistance, and the development of primary resistance models that can be tested with next-generation agents. Only a small fraction of current studies meet this standard, and funding to support re-biopsies and correlative studies remains difficult to procure.
Finally, advocacy for lymphoma research and clinical trial participation is as essential as ever. An ironic consequence of success in the treatment of lymphomas is that the public health need for newer therapies has diminished in comparison with epithelial malignancies, which are not only more common, but also associated with higher mortality rates. During the last two decades, a larger fraction of research funding has come from non-governmental sources, including societies such as the Leukemia and Lymphoma Society, American Cancer Society, and ASH. Supporting the growth of these organizations and maintaining pressure on federal, state, and local governments to support lymphoma research will be essential to accomplish many of the other priorities and thereby to improve the treatment of patients with these diseases.
The published roadmap is simply a template and will mature as new priorities develop and others are accomplished. Another ASH Meeting on Lymphoma Biology is planned for June 2016. Suggestions from the research community, pharma, advocacy groups, and patients for the current roadmap are welcome and can be posted at www.hematology.org/lymphoma-roadmapLymphoma Roadmap.
References
Author notes
https://w.soundcloud.com/player/?url=https%3A//api.soundcloud.com/playlists/105007999&color=b62b30&auto_play=false&hide_related=false&show_comments=true&show_user=true&show_reposts=false;show_artwork=false
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest. Dr. Weinstock is a paid consultant by Novartis and Roche; he receives research support from Novartis.