Study Title:
Risk-Stratified Randomized Phase III Testing of Blinatumomab (IND# 100135, NSC#765986) in First Relapse of Childhood B-Lymphoblastic Leukemia (B-ALL)
ClinicalTrials.gov Identifier:
Sponsor:
National Cancer Institute
Coordinator:
Children’s Oncology Group
Participating Centers:
Multiple sites in the United States and other countries
Accrual Goal:
598 patients
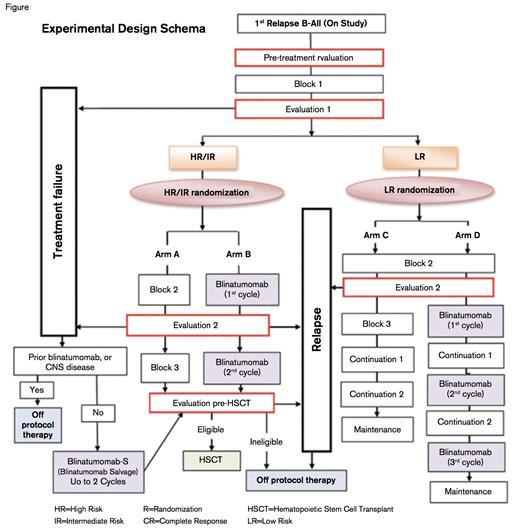
Experimental Design Schema. Trial schema courtesy of Children’s Oncology Group.
Experimental Design Schema. Trial schema courtesy of Children’s Oncology Group.
Study Design:
A risk-stratified randomized phase III trial will test whether the incorporation of blinatumomab into a multi-agent chemotherapy regimen improves disease-free survival (DFS) for patients with a first relapse of B-lymphoblastic leukemia (B-ALL). Patients aged one to 30 years are risk-stratified based on the site and timing of relapse as well as response to a uniform first block of standard re-induction chemotherapy. Subjects are subsequently randomized 1:1 to receive a blinatumomab-containing multiagent chemotherapy regimen versus a control regimen consisting of a multiagent chemotherapy alone. Subjects with treatment failures are non-randomly assigned to receive blinatumomab, and eligible intermediate- and high-risk subjects proceed to hematopoietic stem cell transplantation (HSCT) following three treatment blocks (Figure). Secondary outcomes include rates of negative minimal residual disease (MRD), rates of second morphologic remission, proportion of patients who proceed to HSCT after treatment with blinatumomab, and safety and feasibility of rapid taper of immune suppression following HSCT in subjects with MRD ≥ 0.01 percent pre- and/or post-HSCT with no acute graft vs. host disease (GVHD).
Rationale:
While outcomes for pediatric patients with newly diagnosed B-ALL have improved dramatically in recent decades, outcomes for relapsed disease remain poor. This has prompted efforts to incorporate novel agents into traditional treatment regimens, including several new classes of immunotherapeutics.1,2 Potential advantages of this class of agents include their unique mechanisms of action and non-overlapping toxicity profiles.
Blinatumomab is a bispecific T-cell engager (BiTE®) single-chain antibody construct that directs CD3-positive T cells to CD19-expressing leukemic blasts to effect cell lysis. Preliminary results with this agent have been promising, and the U.S. Food and Drug Administration (FDA) granted accelerated approval for blinatumomab for the treatment of adults with Philadelphia chromosome-negative relapsed or refractory precursor B-cell ALL in December 2014.3 In a recently completed multicenter, single-arm, open-label phase II trial in 189 adults with relapsed or refractory B-ALL (NCT01466179), 43 percent of patients achieved a complete remission or complete remission with partial hematological recovery within the first two cycles of single-agent blinatumomab. Notably, 82 percent of responders achieved MRD negativity (< 10-4 ), and 52 percent who had not had a previous transplant were able to proceed to HSCT.4 Blinatumomab has also been investigated as a single agent in pediatric patients. In a recently completed phase I/II trial, children younger than 18 years of age with a second or greater number of marrow B-ALL relapses, refractory B-ALL, or any marrow relapse after HSCT received blinatumomab by 28-day continuous infusion for up to five cycles.5,6 Notable toxicities included neurological events and cytokine release syndrome (CRS). The MTD was established at 15 µg/m2 /day in the phase I stage of the study. To decrease the risk of CRS, a stepwise dosing schedule of 5 µg/m2 /day for seven days followed by 15 µg/m2 /day was recommended for the phase II portion of the study. Complete remissions were achieved within the first two cycles in 39 percent of children treated with stepwise dosing in both phase I and II (n = 70), and 48 percent achieved MRD negativity, with a median relapse-free survival of 5.2 months. Grade 3 or 4 CRS developed in only 6 percent of patients, and overall side effects were generally reversible.
Based on this demonstrated safety and promising single-agent activity in highly refractory patient populations, the Children’s Oncology Group developed this phase III randomized trial incorporating blinatumomab into a traditional chemotherapy-based regimen for relapsed B-ALL. The majority of patients receive blinatumomab after they have achieved a second remission, thereby reducing the risk of CRS. MRD pre- or post-transplant and the lack of acute GVHD portend a greater risk for treatment failures,7 and this trial also is investigating whether rapid taper of immunosuppression will improve outcomes in these cases.
Comment:
There are several noteworthy features of this trial. In addition to investigating a promising new agent, this trial represents a paradigm shift away from studies designed to test a new agent in small subsets of relapsed ALL patients (e.g., low risk, high risk) using non-randomized historical control outcome comparisons. This trial will improve efficiency for participating centers as it will encompass all B-ALL relapses and the limitations associated with historical outcome comparisons will be avoided. Another unique feature of this trial is the investigation of the feasibility of a post-transplantation intervention (i.e., rapid tapering of immune suppression) in an effort to reduce subsequent relapses among those with the highest risk features. Finally, this trial includes a number of interesting correlative studies that offer promise for refining risk assessment, abrogating toxicities, and predicting response to blinatumomab.
References
Competing Interests
Dr. Raetz indicated no relevant conflicts of interest.