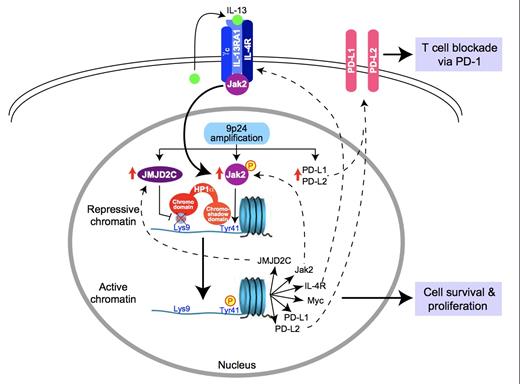
Although the majority of patients with Hodgkin lymphoma (HL) are cured with up-front therapy, the long-term outcome of patients who relapse after autologous stem cell transplantation is poor, despite the availability of brentuximab vedotin (BV). Advances in the understanding of the molecular pathogenesis of HL have led to novel therapeutic targets. Reed-Sternberg (RS) cells are surrounded by an environment rich in inflammatory cells, including lymphocytes, which are not only ineffective in mounting an immune response against the RS cells but also support their growth and survival. Recent studies have identified amplification of chromosome segment 9p, which contains the genes that encode for the programmed death 1 and 2 ligands (PDL1 and PDL2), as the most common genetic aberration in RS cells in the nodular sclerosis subtype of classical HL.The JAK2 gene is also found on chromosome 9p, and increased JAK-STAT signaling results in further up-regulation of PD-1 ligands. PDL1 and PDL2 bind to PD-1 found on T cells, leading to inhibition of activation and proliferation (Figure).
Model for the Pathogenesis of PMBL and Hodgkin Lymphoma. Reprinted by permission from Macmillan Publishers Ltd: Nature Immunology. Rui L, Schmitz R, Ceribelli M, et al. Malignant pirates of the immune system. 12:933-940, copyright 2011.
Model for the Pathogenesis of PMBL and Hodgkin Lymphoma. Reprinted by permission from Macmillan Publishers Ltd: Nature Immunology. Rui L, Schmitz R, Ceribelli M, et al. Malignant pirates of the immune system. 12:933-940, copyright 2011.
Nivolimab, a humanized IgG4 monoclonal antibody directed against PD-1, has been studied in numerous solid malignancies, including melanoma, with promising activity. Given the strong rationale for its possible efficacy in HL, an expansion cohort of HL patients was included in the phase I study. Patients received 3 mg/kg of nivolumab on week 1 and 4, then every two weeks until complete remission, progression, or a maximum of two years. Twenty-three patients were treated, of whom 18 underwent prior autologous stem cell transplantation. Eighteen previously received BV, and 15 received both. All treated patients had a decrease in disease burden, and the overall response rate was 87 percent, consisting of partial and complete response rates of 17 percent and 70 percent, respectively. Among patients who underwent stem cell transplantation and received BV, 87 percent achieved an objective response with 80 percent partial remissions. All three patients who received BV but were transplant naïve achieved a partial remission. The progression-free survival at 24 weeks for all patients was remarkable at 86 percent, and the median overall survival has not yet been reached. In terms of toxicity, the most common adverse events were rash and thrombocytopenia. Twenty-two percent of patients developed grade 3 adverse events, including immune-mediated toxicities of pneumonitis, pancreatitis, and colitis. There were no grade 4 or 5 adverse effects.
Accompanying correlative studies investigated the expression of programmed death ligands. Using FISH assays in 10 available patient samples, three to 15 copies of PDL1 and PDL2 were identified and occurred as a result of amplification, copy number gains, or polysomy of chromosome 9p. In addition, RS cells stained positive for nuclear phospho-STAT3, reflecting active JAK-STAT signaling.
In Brief
Immune checkpoint blockade through the inhibition of PD-1 represents a rational therapeutic approach in HL, with activity in heavily pretreated patients. Further investigation is necessary to determine the durability of responses and immune-related toxicities in this patient population. Additional evaluation of this approach in histologic subtypes other than nodular sclerosis and in EBV-associated HL is also warranted. Moving forward, combining PD-1 inhibitors with BV, as well as other novel agents, holds the promise of highly effective treatment without the use of combination chemotherapy.
Competing Interests
Dr. Ann LaCasce indicated no relevant conflicts of interest.