Chronic Lymphocytic Leukemia and Lymphomas
More than 70,000 new cases of non-Hodgkin lymphoma were diagnosed in the United States during the past year. Despite the introduction of rituximab nearly two decades ago, the majority of patients with lymphoma and chronic lymphocytic leukemia (CLL) who require treatment receive cytotoxic chemotherapy. Recent advances in the understanding of the pathobiology of B-cell lymphoproliferative disorders have led to the identification of multiple therapeutic targets and the development of rational novel agents.
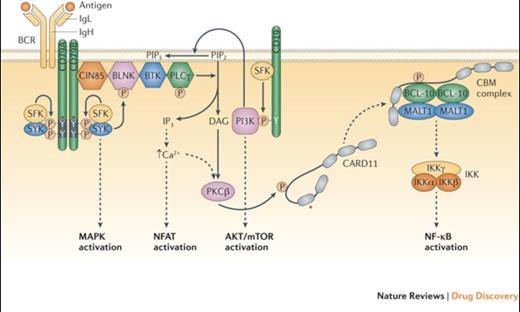
B Cell Receptor Signalling Basics. Shown are the B cell receptor (BCR), the co-receptor CD19, and various signalling intermediates that are engaged following binding of the BCR to antigen. Several downstream pathways are ultimately triggered, as indicated. The asterisk indicates protein regions affected by recurrent somatic alterations in human lymphomas. See main text for details. BLNK, B-cell linker protein; BTK, Bruton tyrosine kinase; CARD11, caspase recruitment domain containing protein 11; CBM, CARD11–BCL-10–MALT1; CIN85, Cbl interacting protein of 85 kDa; DAG, diacylglycerol; IKK, inhibitor of NF-ΚB kinase; IgH, immunoglobulin heavy chain; IgL, immunoglobulin light chain; IP3, inositol trisphosphate; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NF-ΚB, nuclear factor-ΚB; NFAT, nuclear factor of activated T cells; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5- trisphosphate; PKCΒ, protein kinase CΒ; PLCγ, phospholipase Cγ; SFK, SRC family kinase.Reprinted with permission from Nat Rev Drug Discov. 2013;12:229-243.
B Cell Receptor Signalling Basics. Shown are the B cell receptor (BCR), the co-receptor CD19, and various signalling intermediates that are engaged following binding of the BCR to antigen. Several downstream pathways are ultimately triggered, as indicated. The asterisk indicates protein regions affected by recurrent somatic alterations in human lymphomas. See main text for details. BLNK, B-cell linker protein; BTK, Bruton tyrosine kinase; CARD11, caspase recruitment domain containing protein 11; CBM, CARD11–BCL-10–MALT1; CIN85, Cbl interacting protein of 85 kDa; DAG, diacylglycerol; IKK, inhibitor of NF-ΚB kinase; IgH, immunoglobulin heavy chain; IgL, immunoglobulin light chain; IP3, inositol trisphosphate; MALT1, mucosa-associated lymphoid tissue lymphoma translocation protein 1; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; NF-ΚB, nuclear factor-ΚB; NFAT, nuclear factor of activated T cells; PI3K, phosphoinositide 3-kinase; PIP2, phosphatidylinositol-4,5-bisphosphate; PIP3, phosphatidylinositol-3,4,5- trisphosphate; PKCΒ, protein kinase CΒ; PLCγ, phospholipase Cγ; SFK, SRC family kinase.Reprinted with permission from Nat Rev Drug Discov. 2013;12:229-243.
In CLL and mantle cell lymphoma (MCL), Bruton’s tyrosine kinase (BTK), a critical enzyme in the B-cell receptor signaling axis (Figure), mediates the proliferation and survival of malignant lymphocytes through multiple downstream signaling pathways. Ibrutinib, an oral, covalent inhibitor of BTK, received accelerated approval in MCL in late 2013 and in CLL in early 2014. In the pivotal phase II study in relapsed or refractory CLL, the overall response rate (ORR) was 71 percent with a two-year progression-free survival (PFS) of 75 percent.1 A subsequent randomized study comparing ibrutinib to ofatumumab confirmed the drug’s activity with significant improvements in both median PFS and one-year overall survival.2 The activity of ibrutinib was independent of adverse prognostic indicators, including 17p deletion. In mantle cell lymphoma, ibrutinib yielded an ORR of 68 percent, and activity was similar regardless of prior bortezomib exposure.3 The median PFS was 13.9 months, and duration of response was 17.5 months. Additionally, recent preliminary data in both CLL and MCL demonstrated improved activity with the addition of rituximab to ibrutinib.4,5 In terms of toxicity, common adverse events included mild gastrointestinal toxicity and cytopenias. A risk of bleeding, particularly in patients on anticoagulation, and atrial fibrillation, which appears to be related to on-target inhibition of BTK and related kinases in the myocardium, have been reported.1,2,6
Idelalisib, an oral inhibitor of the delta isoform of PI3 kinase, another critical enzyme in the BCR signaling pathway in B-cell lymphomas (Figure), received full approval for use in combination with rituximab in patients considered appropriate for treatment with single-agent rituximab, as well as accelerated approval in relapsed follicular and small lymphocytic lymphomas (SLL). Based on a 72 percent ORR in the phase I study in CLL, a phase III study was conducted comparing rituximab plus idelalisib or placebo in patients with decreased renal function or other major comorbidities.7,8 The ORRs were 81 percent and 13 percent, with 12-month OSs of 92 percent and 80 percent, respectively. In patients with indolent B-cell lymphomas, the majority of whom had follicular lymphoma (FL) or SLL, the single-agent activity in disease refractory to both rituximab and alkylating agents was 57 percent, with 6 percent complete remissions (CRs). The median PFS and duration of remission were 11 and 12.5 months, respectively.9 Therapy was well tolerated, with the most common serious adverse events being neutropenia, transaminitis, diarrhea, and pneumonia.7-9
Moving forward, combining these agents with monoclonal antibodies, as well as immunochemotherapy, will advance the goal of increasing the duration of remissions and OS. In addition, understanding mechanisms of resistance, including the substitution of serine to cysteine at residue 481 of the BTK active site, will lead to improved inhibitors.10
In parallel to the arrival in the clinic of new targeted agents, more data have emerged on underlying driver events in lymphomagenesis, particularly from genome-wide sequencing studies of sequential biopsies. Examination of a series of FL showed that in many cases, early driver mutations were present in chromatin regulator genes such as CREBBP, EZH2, and KMT2D (MLL2).11 This opens the way to novel therapies such as inhibitors of EZH2, with the hope that these agents may be able to deplete the lymphoma progenitor cell pool that gives rise to recurrence.
Modulation of the cellular immune response using checkpoint-blocking antibodies such as anti–PD-1 and anti–CTLA-4 has been successful in numerous epithelial cancers and is now the subject of studies exploring their potential in lymphoma. Two studies of the use of the anti–PD-1 antibody pidilizumab in FL in combination with rituximab,12 and in diffuse large B-cell lymphoma after high-dose therapy13 suggested that this was feasible, but the strongest data to emerge was in Hodgkin lymphoma (HL). In a phase II study in 23 patients with relapsed and refractory HL, most of whom had previously undergone high dose therapy and/or had received brentuximab vedotin, subjects received nivolumab 3 mg/kg every two weeks.14 The response rate in this difficult group was 87 percent, with 86 percent progression-free survival at 24 weeks, 22 percent grade 3 toxicity, and only two withdrawals for this. Although at an early stage, this represents a promising new approach to the treatment of lymphoma, especially in those types such as HL and primary mediastinal lymphoma which are characterized by dysregulation of the PD-1/PD-1 ligand pathway. Finally, in individuals with high risk/multiply-relapsed lymphoma, infusion of expanded autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane antigens produced sustained CRs in a high proportion of patients with minimal toxicity.15 These data indicate that harnessing the immune system to target tumor-associated viral antigens is a feasible approach in heavily pre-treated lymphoma patients, and may have a role earlier in the course of disease.
Acute Lymphoblastic Leukemia (ALL)
Ph-like ALL. The identification of targetable kinase-activating lesions in 10 percent of children to 27 percent of young adults with Philadelphia chromosome-like (Ph-like) ALL is expected to change the treatment paradigm of this poor-prognosis disease. Dr. Kathryn Roberts and colleagues16 identified rearrangements (e.g., ABL1 or ABL2, CSF1R, CRLF2, JAK2, EPOR, PDGFRB, NTRK3) or mutations (e.g., FLT3, JAK1, JAK3, TYK2, SH2B3) in kinase, cytokine, or cytokine receptor genes in 91 percent of patients. In cytokine-dependent cell lines transformed by the various kinase fusions, cell growth was inhibited by the tyrosine kinase inhibitors (TKIs) dasatinib, ruxolitinib, and crizotinib. Forthcoming trials will assess how integration of TKIs with conventional chemotherapy impact response and survival in these patients.
Blinatumomab. Adults with relapsed or primary refractory ALL face a challenging road. Depending on the clinical scenario and patient-specific prognostic factors, salvage chemotherapy can elicit CR rates in the range of 20 to 45 percent with median survivals of three to nine months. Achieving a CR is a first and necessary step in order to realize the curative potential of hematopoietic stem cell transplantation (HSCT). An unmet need clearly exists in this setting.
The bispecific T-cell engager antibody blinatumomab can simultaneously direct lysis of CD19-positive malignant (and normal) B-cells by CD3-positive cytotoxic T cells. In 2014, two phase II trials of blinatumomab in adult patients with refractory/relapsed B-precursor ALL were published.17,18 The initial dose finding and extension protocol in 36 patients by Dr. Max Topp and colleagues17 was followed by a multicenter European and U.S. collaboration that enrolled 189 patients with poor-prognosis features.18 In this study, blinatumomab (administered as a four-week continuous intravenous infusion every six weeks) elicited a CR or CR with partial hematologic recovery (CRh) rate of 43 percent within the first two treatment cycles. Median OS was 6.1 months, and median relapse-free survival was 5.9 months. Blinatumomab elicited minimal residual disease negativity in 82 percent of individuals achieving a CR or CRh; however, the long-term implications of these data in patients with poor-risk disease remain uncertain. The drug was generally well tolerated, with low rates of severe cytokine release syndrome and treatment-associated mortality. Based on the results from the international study, the U.S. Food and Drug Administration approved blinatumomab in December 2014. Moving forward, many questions remain, including the role of combining blinatumomab with conventional chemotherapy in the salvage setting, how to best use the agent as a bridge to HSCT, its efficacy in the frontline ALL population, and what are key predictors of response.
References
Competing Interests
Dr. LaCasce, Dr. Johnson, and Dr. Gotlib indicated no relevant conflicts of interest.