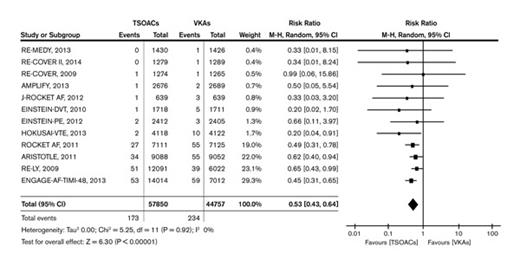
Looking back at 2014, several published articles related to nonmalignant hematology were significant because of their potential to influence clinical practice. Leading the way is the report by Dr. Chatree Chai-Adisaksopha and colleagues, “The Impact of Bleeding Complications in Patients Receiving Target-Specific Oral Anticoagulants: A Systematic Review and Meta-Analysis.”1 By pooling data from 12 phase III randomized, controlled trials involving more than 100,000 patients, the authors compared the direct oral anticoagulants (DOACs) – apixaban, dabigatran, edoxaban, and rivaroxaban – with more traditional anticoagulation strategies such as warfarin. While many hematologists are aware that the DOACs have proven effective in the treatment of venous thromboembolism (VTE) and in the prevention of atrial fibrillation–related stroke, the power to examine the relative safety of DOACs vs. warfarin has been limited by the small number of serious events (e.g., fatal bleeding) that occur in any single trial. The finding from this meta-analysis that fatal hemorrhage occurred less frequently (risk ratio, 0.53; p<0.01) among patients randomized to DOACs may seem surprising because there is no “antidote” for DOACs, while factor replacement and vitamin K can be used to correct warfarin-associated coagulopathy. However, the message from these pooled data is that the ability to “reverse” warfarin did not translate into fewer bleeding-related deaths.
The Forrest plot (Figure) is particularly compelling because the point estimates for relative risk from the individual studies are very consistent: Every one suggests a lower risk of fatal bleeding with the DOACs. Part of this difference is explained by the lower risk of intracranial bleeding associated with DOACs, but the lower risk of fatal bleeding is probably also attributable both to the relatively short half-life of DOACs and to the well-documented grave prognosis associated with major bleeding that occurs on warfarin.2 Regardless of the explanation, the findings of Dr. Chai-Adisakopha et al indicate that for patients with an approved treatment indication, the lack of an antidote for DOACs should not be a rationale for choosing warfarin.
Fatal Bleeding Events. TSOAC = target-specific (a.k.a. direct) oral anticoagulants. VKA = vitamin K antagonists. Reproduced from Chai-Adisaksopha et al.1
Fatal Bleeding Events. TSOAC = target-specific (a.k.a. direct) oral anticoagulants. VKA = vitamin K antagonists. Reproduced from Chai-Adisaksopha et al.1
While the evidence about the relative safety of DOACs is encouraging, the recent “Factor XI Antisense Oligonucleotide for Prevention of Venous Thrombosis” study reminds us that the search for strategies with even lower bleeding risk continues.3 Observations from experiments with animal models and outcome data from humans with congenital factor XI (FXI) deficiency had previously indicated that FXI may be a good target because low levels of FXI activity appear to protect against thrombosis while not increasing the risk of spontaneous bleeding. In this phase II randomized trial of primary VTE prevention after knee replacement surgery, a FXI antisense oligonucleotide (ASO) compared very favorably to a more standard approach with enoxaparin. While this study is too small to permit any definite conclusions about relative safety and efficacy, a critical concept was proven: If given by subcutaneous injection, starting approximately one month prior to surgery, the FXI ASO can migrate to the hepatocyte, decrease FXI synthesis, and provide substantial protection against VTE while conferring a very low risk of major postoperative bleeding.3
Several important articles raised doubts about the benefit of three clinical interventions often considered or recommended by hematologists. From the TIPPS trial, we learned that women with thrombophilia such as protein S deficiency or activated protein C resistance do not benefit from daily prophylaxis doses of dalteparin.4 This multicenter, open-label, randomized trial enrolled 289 women who had a personal history of either thrombosis or pregnancy complications in addition to thrombophilia. The finding that the primary composite endpoint (pre-eclampsia, small-for-gestational-age infant, pregnancy loss, or VTE) occurred with similar frequency in both groups (dalteparin [17.1%; 95% CI, 11.4%-24.2%] vs. no dalteparin [18.9%; 95% CI, 12.8%-26.3%]) corroborates other evidence5-7 and suggests that women with thrombophilia should not be prescribed low-molecular-weight heparin (LMWH) to prevent these complications unless further research indicates that LMWH is beneficial in this setting. Thanks to SOX, a randomized controlled trial to prevent post-thrombotic syndrome in patients with leg deep vein thrombosis, we now know that an elastic compression stocking fitted to achieve 30-40 mmHg pressure at the ankle does not reduce the likelihood that a patient will suffer from symptoms of venous insufficiency more than six months after anticoagulant treatment is begun.8 Finally, 2014 brought us important insights about the challenges of “individualized medicine.” Because of its complex metabolism and its narrow therapeutic index, pharmacogenetic-guided warfarin dosing has been studied extensively ever since genetic variants that impact the pharmacodynamics of vitamin K antagonists were identified. Since individual trials have been small and yielded inconsistent results, it had been difficult to establish whether routinely testing patients for commonly occurring polymorphisms might reduce the risk of clinically important events. After pooling data from nine randomized trials involving 2,812 patients, Dr. Kathleen Stergiopoulos and Dr. David Brown found no benefit from adding pharmacogenetic testing to dosing algorithms that did not use this information: Compared with clinical dosing, the risk ratio for an international normalized ratio (INR) greater than 4 was 0.92 (95% CI, 0.82-1.05) when pharmacogenetic information was added, and the risk ratios for major bleeding and thromboembolic events were 0.60 (95% CI, 0.29-1.22) and 0.97 (95% CI, 0.46-2.05), respectively.9 Based on these observations, it is difficult to justify the addition of pharmacogenetic testing to close follow-up with INR-based dose adjustments.
References
Competing Interests
Dr. Garcia indicated no relevant conflicts of interest.