As hematologists we provide much of the medical care for patients with sickle cell disease (SCD), so we need to remain cognizant that SCD is a truly multisystem disease that affects most tissues and organs beyond the blood and bone marrow. For example, the brain is at ongoing threat of ischemic injury in SCD.1 The risk of overt stroke for children with SCD (without primary stroke prophylaxis) is more than 200-fold higher than the general population, and the burden of stroke may be even higher in adults with SCD.2 As if overt stroke were not worrisome enough, a far more common form of brain injury is silent cerebral infarction (SCI). SCI refers to generally small, permanent brain lesions that do not produce localizing neurologic signs (Figure). The lack of localizing signs should not reassure us, however, because the brain is more than a motor strip. Indeed, SCI is a misnomer because these smaller strokes are often not “silent.” SCI is a morbid condition associated with neurocognitive impairment, poor academic performance, neurologic soft signs, and an increased risk of subsequent overt stroke. SCI can occur as early as the first year of life, and its prevalence increases with age. Approximately 40 percent of adolescents with SCD have SCI, and adults probably continue to acquire new or enlarged SCI lesions.3,4 Despite the high frequency and morbidity of SCI, we have had no randomized clinical trials (with SCI as the primary outcome) to inform medical therapy for patients with SCI, until now.
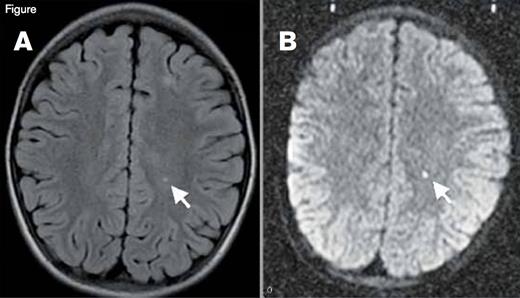
Silent Cerebral Infarction (SCI). A small stroke is visualized here in T2-weighted (panel A) and diffusion-weighted magnetic resonance images (panel B) in a patient with sickle cell disease. Given the restricted diffusion (panel B), this stroke was imaged in the acute phase (onset in the past 10 to 14 days). This patient with incidentally discovered acute silent cerebral ischemia was asymptomatic, had a normal neurologic examination, was in his baseline state (“steady-state”), and was undergoing a screening MRI for a clinical trial. The motor strip was not affected by this stroke, so it is classified as “silent” (an SCI), but a stroke is a stroke, and the brain is more than a motor strip.
Silent Cerebral Infarction (SCI). A small stroke is visualized here in T2-weighted (panel A) and diffusion-weighted magnetic resonance images (panel B) in a patient with sickle cell disease. Given the restricted diffusion (panel B), this stroke was imaged in the acute phase (onset in the past 10 to 14 days). This patient with incidentally discovered acute silent cerebral ischemia was asymptomatic, had a normal neurologic examination, was in his baseline state (“steady-state”), and was undergoing a screening MRI for a clinical trial. The motor strip was not affected by this stroke, so it is classified as “silent” (an SCI), but a stroke is a stroke, and the brain is more than a motor strip.
Among the most important clinical advances for hematologists in 2014, especially those who care for patients with SCD, is the report by Dr. Michael DeBaun and colleagues5 of the results of the Silent Cerebral Infarct Transfusion Trial (SIT Trial). In the SIT Trial, children (ages five to 15 years) with SCD and SCI were randomly allocated to three years of chronic transfusion therapy or observation. Children at high risk of overt stroke were excluded. Chronic transfusions provided a 58 percent relative risk reduction in new or progressive cerebral infarction compared with observation, with a number needed to treat (NNT) of 13. Chronic transfusions also decreased the incidence of other vaso-occlusive complications, but the trade-off was an increased incidence of transfusional iron overload. Given that chronic transfusions can be burdensome and severe iron overload develops in patients who receive inadequate chelation therapy, some clinicians, perhaps put off by the NNT, may balk at implementing this therapy as standard clinical practice. When the Stroke Prevention Trial in Sickle Cell Anemia (STOP) was published in 1998 with a NNT of seven,6 many hematologists then also balked at implementing chronic transfusions for primary stroke prevention, but now it is considered the standard of care. How broadly the SIT Trial results will be implemented by hematologists remains to be seen, but we now have high-quality data from a randomized trial to inform the therapy for patients with SCI, which is still more than can be said for the treatment of most other complications of SCD.
There were many other noteworthy publications in the field of SCD in 2014. Among these, Dr. Matthew Hsieh and colleagues7 reported that adults with severe SCD, who are typically deemed ineligible for hematopoietic stem cell transplantation (HCST), can often safely tolerate a nonmyeloablative regimen with an human leukocyte antigen–matched sibling donor and achieve stable mixed-donor chimerism. These quite promising results with low morbidity and mortality, although not fully mature, suggest that more patients with SCD will be eligible for curative therapy with HSCT in the near future. Two other noteworthy reports highlight novel aspects of the heme-mediated pathophysiology of SCD that mechanistically links hemolysis and vaso-occlusion. Dr. Grace Chen and colleagues8 showed that neutrophil extracellular traps (NETs) and soluble NET components are present in a murine model of SCD, are induced by exposure to free heme, and contribute to vaso-occlusion. Dr. John Belcher and colleagues9 demonstrated that free heme triggers signaling through Toll-like receptor 4, promoting vaso-occlusion through degranulation of Weibel-Palade bodies and expression of adhesion molecules. A final noteworthy report by Dr. Robert Carter and colleagues10 analyzed genomic strains of pneumococci isolated from children with SCD. These investigators identified 60 noncapsular pneumococcal genes (e.g., encoding virulence determinants) that were under differential selective pressure in SCD hosts compared with strains from non-SCD hosts). For example, virulence determinants were distinct from pneumococci isolated from non-SCD hosts, and the protective capacity of potential antigens was lost over time in SCD.
In summary, 2014 provided hematologists and individuals with SCD with high-quality clinical evidence for a new indication for an established therapy (chronic transfusions for SCI), expanded the eligibility criteria for a curative therapy using new techniques (nonmyeloablative HSCT), identified targets for developments of novel drugs for SCD (heme and Toll-like receptor signaling), and informed the development of new vaccines for a potentially fatal complication of SCD (pneumococcal sepsis). The array of work presented at the recent 2014 ASH Annual Meeting indicates that 2015 will also be an exciting year for SCD. As just two examples, we should soon learn more about the role of hydroxyurea for primary overt stroke prophylaxis and the potential role of MEK inhibition in SCD.
References
Competing Interests
Dr. Quinn indicated no relevant conflicts of interest.