The functional capacity of hematopoietic stem cells (HSCs) declines with age, due in large part to the consequences of cumulative DNA damage, with age-related deficiency in DNA repair contributing to acquisition of mutations that lead to bone marrow failure and myeloid malignancies. With the aging of the baby boomer generation, the incidence of myeloid disorders is expected to rise. Therefore, understanding the basis of both age-related bone marrow dysfunction and the origins of myeloid neoplasms is essential for developing effective strategies for addressing these disorders. The recent studies of Dr. Johanna Flach and colleagues in the laboratory of Dr. Emmanuelle Passegué at the University of California, San Francisco, provide novel insights into the mechanisms that underlie age-related decline of HSC function and identify molecular targets for rejuvenation strategies. In experiments that compared outcomes in purified HSCs from young and old mice, those investigators presented data suggesting that replication stress contributes to the chromosomal damage and functional decline characteristic of old HSCs (Figure).
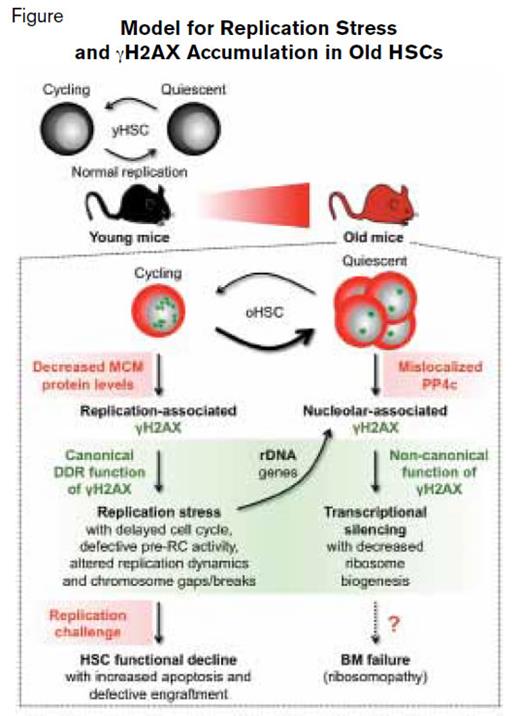
Model for Replication Stress and γH2AX Accumulation in Old HSCs. Reprinted by permission from Macmillan Publishers Ltd: Nature. 512:198-202, copyright 2014.
Model for Replication Stress and γH2AX Accumulation in Old HSCs. Reprinted by permission from Macmillan Publishers Ltd: Nature. 512:198-202, copyright 2014.
The term “replication stress” encompasses a variety of mechanisms that impair DNA copying at replication forks, including delay in cell cycle kinetics, defective prereplication cycle activity, altered replication dynamics, and chromosomal breaks/gaps (Figure). The capacity to circumvent replication stress is central to the maintenance of genomic stability.1 Replication stress is accompanied by phosphorylation of histone H2AX (γH2AX) — an epigenetic mark that serves as the nidus for recruitment of DNA repair proteins (Figure). Dr. Flach and colleagues found evidence of increased replication stress in old HSCs compared with young HSCs during cell cycling that was accompanied by accumulation of γH2AX foci (Figure, small green stars). Subsequent studies identified decreased expression of minichromosome maintenance (MCM) helicase components Mcm 4 and Mcm 6 as the mechanism underlying the greater replication stress observed in old cycling HSCs. The deficiency of MCM components altered replication fork dynamics and impaired progression through the cell cycle, resulting in chromosome gaps and breaks. Nonetheless, old HSCs remain competent in activating canonical DNA damage repair and thereby survive replication unless confronted by a strong replication challenge such as treatment with a replication stressor drug or transplantation (Figure).
To test the relative sensitivity of young and old HSCs, the investigators treated samples with aphidicolin, a DNA polymerase inhibitor that induces replication stress by stalling the copying process at the replication fork. After treatment with aphidicolin, old HSCs had significantly greater γH2AX accumulation and rates of apoptosis compared with treated young HSCs, demonstrating the relative insensitivity of young HSCs to replication stress compared with their old counterparts. However, compared with untreated young HSCs, transplantation of aphidicolin-treated young HSCs resulted in impaired reconstitution capacity, reduced engraftment, and early-onset bone marrow failure and death in transplant recipients — outcomes that were similar to those observed with treated or untreated old HSCs. Thus, forced induction of replication stress in young HSCs recapitulated the aging defect.
Once old HSCs re-established quiescence after cycling, aggregated, residual γH2AX is observed in reformed nucleoli of the postmitotic cells (Figure, large green stars). Because replication stress is intrinsically linked to cell proliferation, the authors sought an alternative mechanism that could account for the large γH2AX foci in quiescent old HSCs. The authors discovered that the persistence of γH2AX in old, quiescent HSCs was due to mislocalization of PP4c, a γH2AX phosphatase (Figure). In this case, PP4c remained in the cytoplasm rather than translocating to the nucleolus where it would be available to dephosphorylate γH2AX. In this setting, persistent nucleolar γH2AX functions noncanonically as a histone modifier, epigenetically silencing transcription of ribosomal DNA genes, resulting in decreased ribosome biogenesis in quiescent old HSCs (Figure). Whether this decreased ribosomal DNA synthesis induced by γH2AX accumulation creates a ribosomopathy that contributes to age-related bone marrow failure remains to be determined (Figure).
In Brief
The studies of Dr. Flach and colleagues suggest that replication stress contributes to the functional decline of old HSCs. Their rigorous studies identified decreased expression of components of the MCM helicase complex as the basis of the replication defect in cycling old HSCs and suggested that ribosomal biogenesis is impaired in quiescent old HSCs due to aberrant epigenetic silencing of ribosomal DNA transcription by γH2AX. This important work identifies decreased expression of MCM genes, and perhaps decreased ribosomal biogenesis owing to mislocalization of PP4c, as molecular targets for pharmacological intervention aimed at rejuvenating old HSC function. Aging of the hematopoietic system has many untoward consequences, such as reduced immune function, anemia, and increased risk of myeloid disorders. Therefore, deciphering the key mechanisms involved in HSC aging and determining how these physiologic changes lead to age-related pathobiology will identify additional new approaches to therapy. As certain chemotherapeutic agents and ionizing radiation induce replication stress in HSC, these results have broader implications for older patients undergoing cancer treatment. Rejuvenation of old HSC function could lead to diminished risk of myeloid disorders and bone marrow failure, or shorten the duration of chemotherapy-induced myelosuppression, and recent parabiosis studies have shown that systemic factors can rejuvenate stem-cell function.2,3 Maybe the fountain of youth is not a myth after all.
References
Competing Interests
Dr. Huang and Dr. Becker indicated no relevant conflicts of interest.