Update/Commentary
Since this article was published there has indeed been a very important development in multiple myeloma (MM) that has implications for IgM monoclonal proteins.
Historically, MM was defined by the presence of “CRAB” criteria (elevated calcium, renal insufficiency, anemia and bony disease). However, three additional criteria have now been added to this definition as endorsed by the International Myeloma Working Group, published in late 2014.1
These three criteria include clonal bone marrow plasma cell percentage of ≥ 60 percent; involved/uninvolved serum-free light chain (FLC) ratio ≥ 100 (involved FLC level must be ≥ 100 mg/L); and more than one focal lesion on magnetic resonance imaging (MRI) studies (at least 5 mm in size).
Each of these criteria was validated by at least two large databases. They do reflect a change in the concept that one must have “established end organ damage” to be treated for myeloma; this is important, as patients with these three criteria have pending organ damage, and if untreated, that damage may be permanent. It has therefore shifted a small subset of smoldering myeloma patients to active myeloma warranting treatment. I remember these three criteria with the acronym “SLiM” (60 percent plasma cells; more than 100 light chains involved/uninvolved; MRI evidence of one or more focal lesions) now creating the overall acronym SLiM CRAB for multiple myeloma.
This new definition of MM, therefore, affects my approach to IgM monoclonal proteins; in the algorithm, I ask the question, “does the patient have end-organ damage attributable to the IgM monoclonal protein?” Instead of only considering CRAB criteria, one should now consider SLiM CRAB criteria, as that would invoke the IgM myeloma diagnosis if present, as opposed to IgM monoclonal gammopathy of undetermined significance (MGUS).
Updated References
The Question
What is your approach to the evaluation of patients with an IgM monoclonal protein?
My Response
Benign monoclonal proteins were first described by Dr. Jan Waldenström in 1960 after he detected abnormal narrow hypergammaglobulinemia bands in serum protein electrophoresis (SPEP) samples from healthy individuals.1 The term monoclonal gammopathy of undetermined significance (MGUS) was coined by Dr. Robert Kyle in 1978 to describe an asymptomatic plasma cell dyscrasia characterized by a monoclonal protein of <3 gm/dL, bone marrow plasma cells <10 percent and the absence of end-organ damage commonly associated with multiple myeloma.2 MGUS is a relatively common condition with a prevalence of three to five percent in the adult population over the age of 50 years.3 SPEP is a frequently performed laboratory test ordered by primary-care physicians evaluating patients with anemia, by nephrologists evaluating patients with renal insufficiency (possibly with associated proteinuria), and by neurologists evaluating patients with peripheral neuropathy. When the SPEP reveals a monoclonal protein, referral to a hematologist usually follows. Therefore, it is part of routine practice for hematologists to see patients with monoclonal proteins that are initially identified as the result of screening assessments. The hematologist must then decide which additional diagnostic studies are warranted, and based upon those results, develop management and follow-up plans.
Although it accounts for only 15 to 20 percent of MGUS cases (that also includes IgG-MGUS, IgA-MGUS and light chain-MGUS), IgM-MGUS poses a unique diagnostic challenge because of the association of monoclonal IgM proteins with B cell lymphoproliferative disorders (particularly Waldenström’s macroglobulinemia, WM), amyloidosis and peripheral neuropathy.4 This review is intended to provide the practicing hematologist with a focused diagnostic approach to patients with a monoclonal IgM protein that takes into account its associations with other disease processes.
MGUS
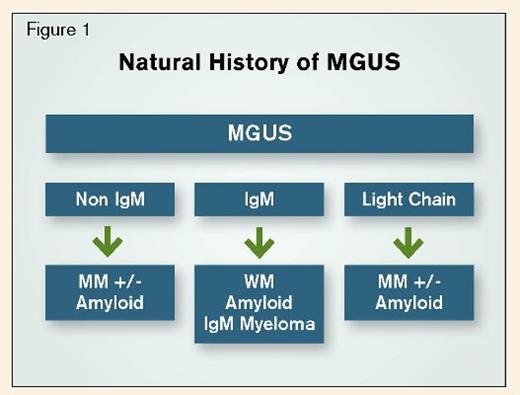
Three distinct classes of MGUS are recognized: Non-IgM-MGUS (essentially IgG-MGUS or IgA-MGUS as both IgD-MGUS and IgE-MGUS are fleetingly rare), light chain-MGUS, and IgM-MGUS.5,6 (Table) Distinguishing among these sub-groups is important as doing so directs both the diagnostic plan and the follow-up recommendations; facilitates identification of diseases associated with MGUS; and impacts on management recommendations and prognosis. Although the majority of patients with IgM-MGUS will have a benign course, it is critical for the clinician to rule out a concurrent associated disease and to monitor for progression or transformation into a distinct entity that requires specific therapy.
The Risk of MGUS Progression
Unstratified, patients with non-IgM MGUS have approximately a one percent per year risk of their disease transforming into multiple myeloma; however, the risk of transformation is double in patients with IgM MGUS. Risk for transformation can be more precisely stratified based on three parameters: IgG subtype vs. non-IgG subtype; M protein concentration <1.5 gm/dL vs. ≥1.5 gm/dL; and normal vs. abnormal serum free light chain ratio.7 Stratification of risk can help guide the diagnostic evaluation and follow-up recommendations.
IgM MGUS not only has a higher risk of transformation than non-IgM MGUS, but also the spectrum of diseases associated with IgM MGUS transformation is broader than that of non-IgM MGUS. Whereas non-IgM MGUS can progress into smoldering and active MM and AL amyloidosis, IgM MGUS can transform into WM, AL amyloidosis, and less commonly, IgM smoldering myeloma or IgM multiple myeloma.8 For this reason, patients with IgM MGUS require closer follow-up than patients with non-IgM MGUS, and from a conceptual perspective, IgM-MGUS can be thought of as a “lymphoproliferative” MGUS while non-IgM MGUS behaves as a “plasma cell proliferative” MGUS.
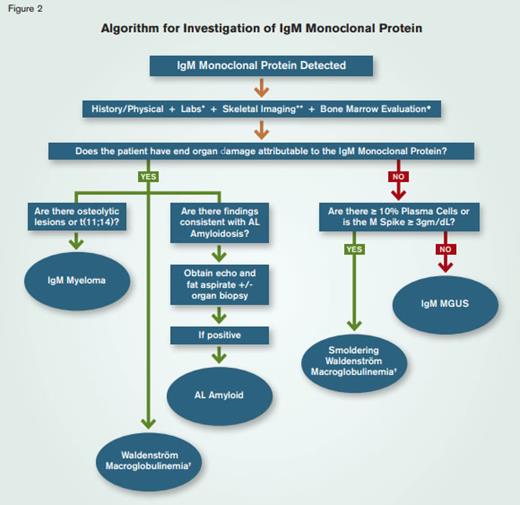
Algorithm for Investigation of IgM Monoclonal Protein.* Laboratory studies include CBC, serum calcium, creatinine, β-2-microglobulin, LDH, liver enzymes, serum protein electrophoresis with immunofixation, serum free light chain assay, quantitative immunoglobulins, 24-hour urine testing for protein, and urine electrophoresis.** Skeletal survey is the preferred first-line imaging choice.◆ Marrow evaluation includes immunohistochemical studies, flow cytometry, conventional cytogenetics, and myeloma FISH.✝ Confirm by MYD88 sequence analysis.
Algorithm for Investigation of IgM Monoclonal Protein.* Laboratory studies include CBC, serum calcium, creatinine, β-2-microglobulin, LDH, liver enzymes, serum protein electrophoresis with immunofixation, serum free light chain assay, quantitative immunoglobulins, 24-hour urine testing for protein, and urine electrophoresis.** Skeletal survey is the preferred first-line imaging choice.◆ Marrow evaluation includes immunohistochemical studies, flow cytometry, conventional cytogenetics, and myeloma FISH.✝ Confirm by MYD88 sequence analysis.
Special Concernsfor IgM MGUS
Association with neuropathy – Neurologists routinely screen patients with peripheral neuropathy for the presence of a monoclonal protein, and approximately five to 10 percent of such patients will be found to have an M protein by SPEP. A causal relationship between MGUS and peripheral neuropathy is supported by association of peripheral neuropathy with other plasma cell dyscrasias including WM, MM, AL amyloid and POEMS (polyneuropathy, organomegaly, endoocrinopathy, monoclonal gammopathy, skin changes). The peripheral neuropathy of MGUS is classically bilateral, peripheral and sensory with electrophysiologic studies showing a demyelinating pattern; and in patients with progressive, debilitating disease, biopsy may reveal axonal loss. Although peripheral neuropathy can occur with all forms of MGUS, it is most commonly associated with IgM-MGUS. Demonstration of IgM anti-myelin-associated glycoprotein (MAG) antibodies supports a causal relationship between IgM-MGUS and polyneuropathy but is not essential for the diagnosis. Because peripheral neuropathy can be caused by other processes that may co-exist with IgM-MGUS, the clinician is often faced with the dilemma of whether to assign the neuropathy to MGUS, and if so, what to do about it. The decision can be guided by the observations that the neuropathy associated with IgM-MGUS is characteristically a relatively benign, slowly progressive sensory process (although some cases can be severe and debilitating). Management is challenging as effective therapy is lacking. A minority of patients respond to rituximab and other immunomodulatory treatments are generally ineffective.9 As is the case with other plasma cell dyscrasia-associated neuropathies, the M protein concentration in IgM-MGUS-associated peripheral neuropathy does not correlate with disease severity, arguing against the use of myeloma-directed therapy to reduce the plasma cell burden as a treatment strategy for the neuropathy of IgM-MGUS.
Association with AL amyloid – This complex disease can be associated with any form of myeloma, although usually one of the two processes dominates the clinical picture. That is to say that patients typically have either a myeloma-phenotype manifested by some combination of hypercalcemia, renal insufficiency, anemia, and skeletal involvement or an amyloid phenotype characterized by organ (liver, heart, kidney) infiltration by the pathologic immunoglobulin light chain. AL amyloid is more commonly associated with IgM-MGUS than other forms of MGUS. Thus, it is imperative that AL amyloid be carefully considered when evaluating patients with an IgM monoclonal protein.
Association with WM – The hallmark of this disease is an IgM monoclonal protein, lymphoadenopathy, hepatosplenomegally, and bone marrow involvement by plasmacytoid lymphocytes. Frank disease may be preceded by a relatively asymptomatic smoldering phase in which IgM MGUS is the only apparent clinical manifestation.
Association with other B-cell lymphoproliferative disorders – Although IgM-MGUS is more commonly associated with WM, it can be observed in association with another B cell lymphoproliferative disease such as CLL or non-Hodgkin lymphoma. As indicated in the algorithm (Figure), determining if organ damage is attributable to the M protein is critical, as in most cases the monoclonal protein simply “co-exists” with the lymphoproliferative disorder. Although the M protein may be discovered first as part of a laboratory evaluation, it is not usually the presenting clinical manifestation of lymphoproliferative diseases other than WM.
Association with IgM MM – This is a rare form of myeloma that is genuinely distinct from WM. IgM MM is distinguished from WM primarily by skeletal involvement (lytic bone lesions) in the former, but genetic studies may also be informative [e.g., t(4:14) in IgM MM and somatic mutation of MYD88 in WM].10,11
Suggested Approach to the Evaluation of Patients with an IgM M Protein.
The strategy that I use in the evaluation of patients with an IgM monoclonal protein is described below and illustrated in the figure.
History – This remains a critical aspect of the evaluation, as symptoms elicited from a careful history focus attention on specific issues that require further investigation. Indeed, even subtle symptoms become important when the differential diagnosis includes such a wide spectrum of disorders as amyloidosis, POEMS, multiple myeloma, and lymphoma. Key symptoms to address include the following:
Constitutional (weight loss, extreme fatigue) – Such symptoms suggest AL amyloid or lymphoma.
Gastrointestinal – Upper GI bleeding, early satiety, and chronic diarrhea raise the possibility of GI amyloid.
Cardiac – Progressive shortness of breath, presyncope/syncope and chest pain are consistent with cardiac amyloid.
Neurological – Bilateral, sensory neuropathy is consistent with the neuropathy associated with plasma cell dyscrasias; and vision changes, headache, vertigo or dizziness raise the possibility of hyperviscosity associated with WM.
Skeletal pain – This symptom suggests IgM myeloma.
Skin – Urticarial rash raises the possibility of Schnitzler syndrome.
Physical Exam – The physical exam can be informative as patients with an IgM M protein and WM or other non-Hodgkins lymphoma may present with lymphadenopathy and heptosplenomegaly. Patients with concurrent AL amyloid may have hepatosplenomegally or macroglossia, while patients with IgM myeloma may have discrete sites of skeletal pain. A careful neurological exam is essential for identifying and characterizing a concurrent neuropathy.
Labs
The following lab studies should be obtained:
CBC, serum calcium, creatinine, β-2-microglobulin, LDH, liver enzymes
Serum protein electrophoresis with immunofixation electrophoresis
Serum free light chain assay
Quantitative immunoglobulins
Spot urine sample for protein quantitation, protein electrophoresis and immunofixation electrophoresis. For patients with significant proteinuria and patients with a monoclonal light chain (Bence Jones protein) detected in the spot urine, a 24-hour urine sample should be collected to quantitate total protein and light chain excretion.
Other laboratory tests that should be considered based on the clinical circumstances:
Cardiac function biomarkers (NT-proBNP and troponin) for suspected amyloid
Anti-MAG antibodies in patients with a sensory neuropathy
Peripheral blood flow cytometry for suspected CLL
Radiologic and Other Diagnostic Testing
Skeletal survey (radiographic or MRI myeloma protocol) is recommended for patients with bone pain and for patients in whom IgM MM is a consideration.
Others diagnostic studies that may be considered based on clinical circumstances:
Abdominal US to assess for hepatic amyloid
Echocardiogram and ECG to assess for cardiac amyloid
Abdominal/pelvic CT to assess for non-Hodgkin’s lymphoma including WM
PET scan to assess for lymphoma
Bone marrow evaluation – Although many patients with MGUS do not require this procedure, in higher-risk cases (M-protein concentration >1.5 gm/dL and IgM-MGUS), bone marrow aspirate and biopsy is recommended and may also include cytogenetic, myeloma FISH, and flow cytometric analyses and targeted gene sequencing.13 I do not, however, recommended bone marrow analysis for all patients with an M protein. Specifically, I may defer bone marrow analysis in the very elderly, in those in whom the M protein concentration is low (less than 0.5 gm/dL), and in patients with an inflammatory condition, as low risk MGUS is relatively common in these settings.
Myeloma FISH and targeted gene sequencing – Recommended if marrow findings are consistent with MM or lymphoma. Finding t(11;14) by cytogenetics or FISH suggests IgM MM, and mutant MYD88 supports a diagnosis of WM.
Flow cytometry – This analysis is informative in patients suspected of having a B cell lymphoproliferative disorder including CLL, non-Hodgkin lymphoma or WM.
Fatpad aspirate – I do not routinely subject patients to this procedure, however, if there is clinical suspicion of AL amyloid, fat pad aspirate with congo red staining is an excellent screening tool. I have a slightly higher index of suspicion for AL amyloid when the IgM M protein is lambda-restricted. If the fat pad aspirate is negative for AL amyloid but suspicion remains high, target organ biopsy with congo red staining is warranted.
Follow-Up
If after the initial evaluation a patient is diagnosed with IgM MGUS, regular surveillance is imperative as risk of progression/transformation is a continuous variable. I recommend that patients be seen twice annually with a clinical assessment, along with the following laboratory studies: CBC, comprehensive metabolic panel, SPEP, serum-free light chain assay, and quantitative immunoglobulins.14 I do not recommend repeat imaging or bone marrow analysis unless there is suspicion of progression.
Conclusion
Monoclonal IgM is associated with a diverse set of diseases that range from a generally benign process requiring no specific therapy (IgM-MGUS) to overt disease requiring a specific management plan. Approaching the initial evaluation systematically and comprehensively will enable the clinician to accurately characterize the disease process.
References
Author notes
The update/commentary section was added in 2016 when this article was included in the Ask the Hematologist Compendium 2010-2015Ask the Hematologist Compendium.
Competing Interests
Dr. Mikhael declares no relevant conflicts of interest.