The following is excerpted from and based on an article published by the author in the Transactions of the American Clinical and Climatological Association (Trans Am Clin Climat Assoc. 2010;121:61-73) with permission.
Authors' Note: This paper is dedicated to those hemophiliacs who died of AIDS, to Harold R. Roberts, MD, and Campbell McMillan, MD, mentors who taught me commitment and compassion in the treatment of all human conditions, and to Martha Warren Turvey, Aime Beebee, and Brenda Nielson, nurses at the Harold R. Roberts Comprehensive Hemophilia Diagnosis and Treatment Center at the University of North Carolina, Chapel Hill, truly remarkable individuals who provided the very highest possible care in the very worst possible times.1
Prior to the 1960s, the average life expectancy for an individual diagnosed with hemophilia was 11 years. When I came into hematology in the mid-1970s, treatment of hemophilia had been greatly improved by advent of the availability of glycine-precipitated plasma concentrates and, consequently, the average lifespan of an individual diagnosed with hemophilia had increased to about 42 years. But life for patients with hemophilia remained challenging as treatment often meant a trip to the emergency room followed by an inpatient admission; hepatitis B and a non-A, non-B form of blood-borne hepatitis (that we now know was hepatitis C) were starting to appear at a high frequency among patients; hemophiliacs who developed inhibitory antibodies had no treatment options; and joint dysfunction and deformity continued to plague the population.
What has happened in the 40 years since then is a story not only of terrible tragedy but also of remarkable accomplishment that has led us to the threshold of a cure. Ultimately, though, it is a compelling story of the power of medical research that is driven not only by curiosity but also by passion and of the resiliency of patients and their caregivers, who persevered in the face of overwhelming adversity and, in the end, triumphed.
The Tragedy – AIDS in Hemophilia
The availability of glycine-precipitated factor VIII concentrates enabled hemophiliacs, for the first time, to initiate treatment at home. This treatment option opened up a whole new world in which hemophiliacs could treat themselves more rapidly, thereby limiting the severity of hemarthroses and other bleeding complications, reduce dependency on emergency room and inpatient treatment, treat prophylactically prior to activities that might cause bleeding, travel, and have safe surgery for joint disease and other disease- and non-disease-related problems. But all of that progress ended abruptly in 1982 with the first report of AIDS in hemophilia patients.1 Over the course of the next few years, almost 5,000 hemophiliacs in the United States were found to be infected as a result of transmission of HIV through plasma-derived treatment products, and more than 4,000 of the estimated 10,000 hemophiliacs in the United States would eventually die of AIDS. These were dark times for the hemophilia community. The freedom that patients had gained from the availability of factor concentrates disappeared. Patients stopped treatment, and there was confusion among physicians about how to manage patients. Patients that I and the other members of the hemophilia team in Chapel Hill had followed for years began to die of complications related to AIDS. Because the treatment of hemophilia is from birth to death, the staff of a hemophilia center becomes unusually attached to their patients and the patients to the staff. We knew their goals and aspirations; we had seen how their goals had to be modified by their hemophilia and now, with AIDS, we saw that their goals might never be achieved.
The advent of the AIDS epidemic, however, drove scientific discoveries that ironically have now improved the treatment of hemophilia. These discoveries were fueled by the tragedy that was happening in the hemophilia community and driven by a robust collaboration among academic medical centers, the then fledgling biotechnology industry, and pharmaceutical companies. In retrospect, these discoveries came rapidly, but they did not come fast enough to save the lives of nearly half of the U.S. hemophilia population.
Cloning the F8 Gene and the Development of Recombinant Products
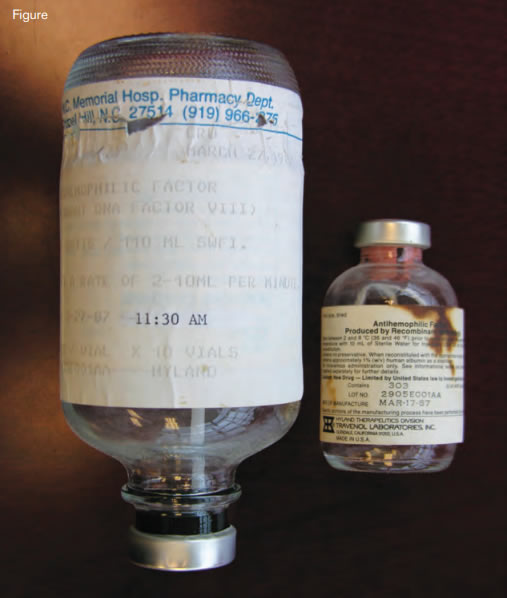
Material Used in the First Treatment With Recombinant FVIII (rFVIII) of a Patient With Hemophilia A. The infusion set used on March 27, 1987, in the General Clinical ResearchCenter at the University of North Carolina, Chapel Hill, is shown on the left. The vial containing the first clinical lot (#2905E001AA1) of rFVIII manufactured on March 17, 1987, is shown on the right (copyright G. C. White, II).
Material Used in the First Treatment With Recombinant FVIII (rFVIII) of a Patient With Hemophilia A. The infusion set used on March 27, 1987, in the General Clinical ResearchCenter at the University of North Carolina, Chapel Hill, is shown on the left. The vial containing the first clinical lot (#2905E001AA1) of rFVIII manufactured on March 17, 1987, is shown on the right (copyright G. C. White, II).
The first of the landmark discoveries that changed the management of hemophilia was the cloning of the F8 and F9 genes and the generation of recombinant proteins for therapeutic use. Two groups, one at Genetics Institute in Boston and one at Genentech in South San Francisco, cloned the F8 gene simultaneously.2-5 Once cloned, the race was on to develop recombinant forms of factor VIII. Just three years later, in March of 1987, the first infusion of recombinant factor VIII was given at our Hemophilia Center at the University of North Carolina (Figure).6 The individual selected to receive the product was a highly articulate 43-year-old man. While obtaining his informed consent, I explained to him that the product had been generated in Chinese hamster ovary (CHO) cells and that one of the concerns was that, being produced in a non-human cell, the factor VIII might not fold properly and antibodies might develop. I explained that by all methods of comparison, the CHO-produced protein was identical to the protein isolated from human plasma, but antibody formation was still a potential risk. On the day of the initial infusion, there was a news crew in the General Clinical Research Center to record the historic occasion. The lights were bright, and I was nervous. As I was infusing the material, I noticed that the patient was sitting with his eyes closed with his chin resting on his chest. I asked if he was okay, but he did not answer. I asked again, but again no answer. Louder, I said, “speak to me.” He looked up at me and started making hamster noises, intending to suggest that the infusion of a product synthesized in hamster cells had transformed him into a hamster. After the product was licensed, the patient was invited to Genetics Institute Headquarters in Cambridge, Massachusetts, for a celebration. During the festivities, Gabe Schmergel, the CEO of Genetics Institute, invited the patient to make some comments. After slowly and painfully climbing to a balcony half way up the stairs, he delivered a powerful story about what it was like to grow up with hemophilia without adequate treatment, how as a child he had lost a beloved older brother as the result of a bleed, and how important the development of safe recombinant factor was to him and all people with hemophilia; for the employees at Genetics Institute, his comments made their work relevant in an unforgettable way. Humor, selflessness, and undaunted spirit in the face of adversity were the signature characteristics of the hemophilia population during the dark days of the AIDS era, but there was also anger.
Today, more than 88 percent of the factor administered in the United States is a recombinant product, free of all human proteins.
Genetic Engineering and Molecules of the Future
One of the great promises of recombinant DNA technology is its use to reduce the cost of treatment through improvements both in the efficiency of FVIII and FIX production and by engineering functionally enhanced molecules, especially those with a longer half-life in circulation. The cloning of F8 and F9was a critically important step in developing genetically improved molecules as the availability of large quantities of FVIII and FIX made feasible crystallization of portions of these proteins that allow determination of their molecular structure.7-8 Cloning and expression of factors VIII and IX also permitted mutagenesis studies, which provided essential information about function. Together, these studies have resulted in an unprecedented understanding of the structure and function of FVIII and FIX, serving as the foundation for the current success in generating recombination and derivatized proteins with favorable therapeutic properties (Table).
Table. Characteristics of Next-Generation Products for Treatment of Hemophilia
| Prolonged circulation . |
|---|
| Polyethylene glycol derivatized recombinant proteinsImmunoglobulin or albumin fusion moleculesTargeted inhibitors of degradation |
| Decreased rate of inactivation . |
| Modified sequences that mediate inactivationModified sequences to inhibit subunit dissociation |
| Increased clotting factor activity . |
| Modified sequences to increase catalytic activity |
| Increased efficiency of synthesis of clotting factors . |
| Improvements in biomedical engineering |
| Prolonged circulation . |
|---|
| Polyethylene glycol derivatized recombinant proteinsImmunoglobulin or albumin fusion moleculesTargeted inhibitors of degradation |
| Decreased rate of inactivation . |
| Modified sequences that mediate inactivationModified sequences to inhibit subunit dissociation |
| Increased clotting factor activity . |
| Modified sequences to increase catalytic activity |
| Increased efficiency of synthesis of clotting factors . |
| Improvements in biomedical engineering |
A significant limitation of the current recombinant products is their rate of clearance. The half-life of FVIII is approximately 12 hours and that of FIX is somewhat longer. The clearance of FVIII seems particularly complex. FVIII binds to von Willebrand factor but also appears to interact with multiple receptors such the low-density lipoprotein receptor-related protein.9 Conceptual approaches to develop clotting factor concentrates with prolonged half-lives and superior performance are listed in the Table. All of these advances in knowledge about the structure, function, production, and catabolism of FVIII and FIX are the direct outcome of the cloning of those proteins, a feat of scientific ingenuity fueled by the tragedy of AIDS.
HIV-1 and the Prospect for Gene Therapy
The story of hemophilia and AIDS can be seen as an allegory of the resilience of the human spirit, and embedded in this story is an ironic twist of fate. The relationship between hemophilia and AIDS played an important role in the identification of HIV-1 as the causative agent of AIDS, but it was the subsequent characterization of HIV-1 that suggested that the retrovirus that killed nearly half of the hemophilia population in the United States had properties that might make it a candidate to deliver a cure for the disease. At the same time that substantial headway was being made in the treatment of AIDS by the development of anti-retroviral therapeutics, retroviruses, first Moloney murine leukemia virus and later lentiviruses including HIV, were being developed as gene transfer vectors.10 Characteristics that made them appealing gene transfer agents were their efficient entry into cells; their ability to integrate into the chromosome, albeit in a random manner; the ability to pseudotype the virus to target it to specific cells; the ability to render the virus replication-defective through use of packaging systems; and the relatively small size of the virus genome. Thus, the virus that had caused so much suffering and death in the hemophilia population had the potential to favorably modify the natural history of the disease. The first subject I enrolled in a gene transfer study was a 51-year-old patient with hemophilia A who had severe joint disease and who was blind in one eye as a result of an accident complicated by bleeding. He was keen to participate in the trial because he had two affected grandsons, and he wanted a better life for them. This was a trial using a Moloney-based retrovirus containing the cDNA for factor VIII. The patient tolerated the treatment well. Factor VIII levels up to 1.7 percent were observed, and a reduction in bleeding frequency was documented, but overall, the trial did not show a dose-response effect sufficient to move this approach forward as a therapeutic. But the quest for a cure continues, and a recent study, using self-complementing adeno-associated virus type 8 to target codon-optimized human factor IX to liver, showed persistent factor expression of up to 11 percent.11
Conclusions
At the outset, I indicated that this is ultimately a story of the power of medical research and the remarkable response by scientists to a tragedy. It is a story that needs to be told; it needs to be told to those who think that our research isn’t translational enough, to those who fund research, because it illustrates what research can accomplish, and to students, so they can see the value of research. After the advances of the past 40 years, the average life span of a hemophiliac today is more than 60 years, nearly that of a non-hemophiliac male. And the quality of life is considerably improved. There have been no new cases of product-transmitted hepatitis C or HIV in well over a decade, and many of the patients who survived to see the development of protease inhibitors remain alive today. The availability of safe replacement therapy has re-kindled efforts to implement primary prophylaxis treatment for children and adolescents and, in some countries, adults.
But, this is not the end of this story; it remains a story in evolution. I am optimistic that the treatment of hemophilia in 40 years will look quite different from that of today, highlighted by the availability of longer-lasting concentrates that mean treatment is needed only once a month and by development of concentrates that are inexpensive enough that patients can be treated for their entire life. Or perhaps oral forms of factor VIII and factor IX will be developed. Progress in gene transfer technology will undoubtedly continue such that we may soon talk realistically about a cure. The remarkable scientific advances of the past 40 years hold out the promise that all of these things will be a reality in the next 40. I am grateful to have been a part of this story of sacrifice and triumph, and I cannot and will not forget those who were involved.