Autophagy was first described in 1963 by Nobel laureate Christian de Duve as a morphologic process in which cellular components are internalized and degraded by lysosomes.1 Three types of autophagy are now recognized: 1) Macroautophagy, the type described by de Duve, involves engulfment of cytoplasmic contents by an organelle called the autophagosome. Macroautophagy is a complex process that is orchestrated by a large group of autophagy-related proteins known as ATGs; 2) microautophagy refers to the direct engulfment of cytoplasm by the lysosome; and 3) chaperone-mediated autophagy targets individual proteins. Autophagy plays a fundamental role in a variety of cellular events, including metabolism, immunity, and cellular repair. However, recently, macroautophagy also has been implicated in protein secretion. For example, deletion of a critical autophagy gene, Atg7, within pancreatic beta cells leads to impaired secretion of insulin.2
Dr. Takehiro Torisu and colleagues in the laboratory of Dr. Toren Finkel at the Center for Molecular Medicine at the National Heart, Lung, and Blood Institute in Bethesda, Maryland, now find that macroautophagy is involved in the mechanisms that regulate expression of von Willebrand factor (vWF) by endothelial cells. vWF is the major component of Weibel–Palade bodies3 and is produced by a complex biosynthetic pathway that includes dimerization of pro-vWF monomers in the endoplasmic reticulum, furin-dependent cleavage of pro-vWF, and multimerization of mature vWF in the Golgi complex.4
By use of immunoelectron and immunofluorescence microscopy, the authors observed that Weibel–Palade bodies and autophagosomes are located in cultured human umbilical vein endothelial cells (HUVECs) in closer proximity than expected by chance. Additionally, they observed that Weibel–Palade bodies and autophagosomes are occasionally found fused together. Additional immunoelectron microscopy using anti-vWF antibodies revealed the presence of vWF in autophagosomes. Lentiviral shRNA-mediated knockdown of the autophagy genes, Atg7 and Atg5, in HUVECs produced several abnormalities on vWF expression. Although the intracellular concentration of vWF was not reduced, the ratio of pro-vWF to mature vWF was significantly increased in Atg7 or Atg5 knockdown cells. Stimulated release of vWF by histamine or vascular endothelial growth factor was reduced. Two inhibitors of autophagosome function, chloroquine and bafilomycin, blocked stimulated release of vWF from HUVECs. Additionally, the number and size of Weibel–Palade bodies was decreased in Atg7 and Atg5 knockdown cells, as well as in cells treated with chloroquine or bafilomycin. The rate of exocytosis of Weibel– Palade from endothelial cells was also decreased in Atg7 knockdown cells compared with normal HUVECs.
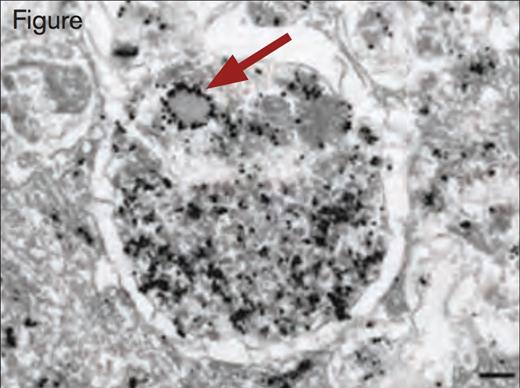
Immunogold Detection of vWF Protein in a Weibel–Palade Body Within an Autophagosome. Scale bar, 200 nm. Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine. 2013; 19(10):1282, Copyright 2013.
Immunogold Detection of vWF Protein in a Weibel–Palade Body Within an Autophagosome. Scale bar, 200 nm. Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine. 2013; 19(10):1282, Copyright 2013.
The authors developed an in vivo model to assess the role of autophagy in vWF expression by endothelial cell-specific deletion of the Atg7 gene in mice using the Cre recombinase system. Vascular development and structure in these mice, called Atg7endo mice, were normal, as judged by examination of skeletal muscle and retinal microvasculature and large vessel anatomy. However, cultured endothelial cells from Atg7endo mice displayed reduced numbers of Weibel–Palade bodies. Although plasma vWF levels were normal in Atg7endo mice in contrast to control mice, there was no epinephrine-induced increase in plasma vWF in Atg7endo mice. Additionally, plasma vWF from Atg7endo mice expressed lower levels of higher-molecular-weight vWF multimers compared with control mice. Consistent with this finding, the tail snip bleeding time was prolonged in Atg7endo mice compared with control mice.
In Brief
The results of this study provide additional evidence that autophagy is involved in the regulation of protein expression in certain specialized cell types, including endothelial cells. Exocytosis from endothelial cells, including release of vWF, provides a major defense mechanism after vascular injury. Elucidation of the role of autophagy in vWF expression adds a new dimension to the regulation of this complex protein and may have implications for the pathogenesis of von Willebrand disease.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.