In the Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial,1 the safety and efficacy of unmonitored dabigatran in more than 18,000 patients was examined. The trial was designed to establish non-inferiority of two different dose levels of dabigatran compared with warfarin for prevention of stroke or systemic embolism in patients with nonvalvular atrial fibrillation and one additional risk factor for thrombosis. After a median follow-up of two years, patients receiving dabigatran, 110 mg twice daily, had similar rates of stroke or embolic events and lower rates of major hemorrhage compared with the warfarin-treated control group. At the higher dose level of 150 mg twice daily, the investigators found lower rates of stroke and systemic embolism and similar bleeding rates compared with the warfarin group. On the basis of these data, the FDA approved dabigatran for reduction of stroke and systemic embolism in nonvalvular atrial fibrillation in 2010, thereby providing patients and clinicians the first oral, unmonitored alternative to warfarin. At the same time, pharmacokinetic analyses revealed wide inter-individual variation in dabigatran plasma concentrations.2 While the RE-LY trial demonstrated the promise of unmonitored dabigatran, there remained a deficit of data on whether and how variability in plasma drug concentrations might impact clinical outcomes. In the present study, Dr. Paul Reilly and colleagues aimed to address this important issue.
The study examined the correlation between plasma concentrations of dabigatran and the clinical outcomes of ischemic stroke/systemic embolism and major bleeding among a subset of patients who had been enrolled in the RE-LY trial. Plasma dabigatran levels were measured using tandem mass spectrometry at peak (1 to 3 hours post-treatment) and trough (10 to 16 hours post-treatment) in subjects who had been taking dabigatran for one month. The impact of clinical and demographic variables was included in the investigation.
Using plasma samples from 9,183 patients, the authors found that dabigatran concentration was influenced by renal function, age, weight, and gender. Plasma concentrations showed greater than five-fold variation between the 10th and 90th percentile at both peak and trough for both the 110 mg and the 150 mg dose, and a large overlap in drug levels was observed between the two doses.
Trough dabigatran concentration was an independent predictor of both ischemic and hemorrhagic outcomes. Multiple logistic regression modeling showed that trough dabigatran levels were inversely related to the probability of an ischemic event (p=0.045) and directly related to the risk of major bleeding (p<0.0001). The best-fit model to predict major bleeding risk included trough dabigatran concentration, age, aspirin use, clopidogrel use, sex, and coronary artery disease. In multivariate analysis of ischemic stroke/systemic embolism risk, the best-fit regression model included trough plasma concentration, age, previous stroke/transient ischemic attack, and diabetes mellitus.
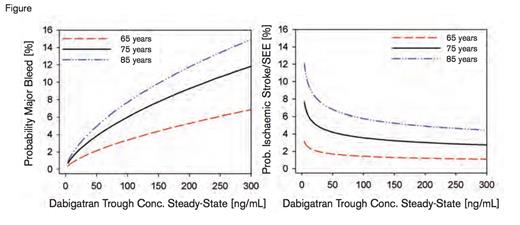
Probability of Clinical Outcomes Versus Dabigatran Plasma Concentrations. Reprinted from the Journal of the American College of Cardiology, Vol. 63, Reilly PA et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), Page 325, Copyright 2014, with permission from Elsevier.
Probability of Clinical Outcomes Versus Dabigatran Plasma Concentrations. Reprinted from the Journal of the American College of Cardiology, Vol. 63, Reilly PA et al. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (Randomized Evaluation of Long-Term Anticoagulation Therapy), Page 325, Copyright 2014, with permission from Elsevier.
The figure illustrates the probability of major bleeding and ischemic events as a function of dabigatran trough plasma concentration and patient age. The probability of ischemic events flattened out at trough levels above 100 ng/mL but increased steeply with lower concentrations. In contrast, the probability of major bleeding continued to increase in a near linear fashion at trough concentrations above 100 ng/mL. These data suggest that in patients with relatively high trough dabigatran levels, decreasing the dose to target a concentration of ~100 ng/mL could reduce bleeding risk without appreciable loss of efficacy. Conversely, in patients with relatively low trough levels, increasing the dose to target a concentration of 100 ng/mL could reduce ischemic events at the potential cost of increased bleeding.
In Brief
The relationship between trough dabigatran levels and ischemic and hemorrhagic events presented by Dr. Reilly and colleagues suggests a potential role for monitoring dabigatran to guide dosing and thereby optimize clinical outcomes. A randomized, controlled trial comparing dose-adjusted with standard unmonitored dabigatran is needed to test this hypothesis. Several major questions need to be tackled in designing such a trial. First, should all patients meeting RE-LY eligibility criteria be included, or should enrollment be restricted to patients with advanced age, renal dysfunction, or other characteristics that influence drug concentration? Second, should dabigatran levels be measured at just one time point (as in the study by Reilly et al.), or should they be measured more frequently (as with warfarin)? Third, should monitoring be carried out using tandem mass spectrometry (as in the study by Reilly et al.), or should a functional coagulation assay such as the dilute thrombin time, which may be more practical in a clinical setting, be used?3 Fourth, how would monitoring affect cost-effectiveness of dabigatran? Similar questions regarding the relationship between clinical outcomes and levels of the heretofore unmonitored oral factor Xa inhibitors, rivaroxaban, apixaban, and edoxaban, must also be asked. While we await answers to these questions, the data presented by Dr. Reilly and coworkers serve as a reminder that we still have much to learn about optimal clinical implementation of the novel oral anticoagulants. This class of drugs is likely to greatly simplify therapy for many patients, but a no-monitoring paradigm must be viewed with caution.
References
Competing Interests
Dr. Villa and Dr. Cuker indicated no relevant conflicts of interest.