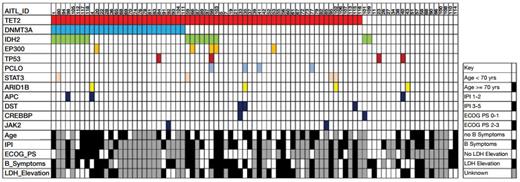
Distribution of Mutations in 85 Cases of AITL. Common mutations and demographic and clinical factors across the cohort are shown. Each column represents a single patient. IPI = International Prognostic Index; ECOG PS = European Cooperative Oncology Group Performance Status.From Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T cell lymphoma. Blood. 2014;123:1293-1296.
Distribution of Mutations in 85 Cases of AITL. Common mutations and demographic and clinical factors across the cohort are shown. Each column represents a single patient. IPI = International Prognostic Index; ECOG PS = European Cooperative Oncology Group Performance Status.From Odejide O, Weigert O, Lane AA, et al. A targeted mutational landscape of angioimmunoblastic T cell lymphoma. Blood. 2014;123:1293-1296.
Though angioimmunoblastic T-cell lymphoma (AITL) is a rare disease, it is one of the more common subtypes of peripheral T-cell lymphoma in the Western world. Patients typically present with advanced-stage disease and systemic symptoms, and associated autoimmune manifestations of the disease are common. Histologically, AITL is characterized by a proliferation of malignant T cells in a mixed background of polyclonal plasma cells, eosinophils, histiocytes, and B cells, often harboring the Epstein- Barr virus (EBV), in an expanded network of follicular dendritic cells with increased numbers of high endothelial venules. The pathogenesis of the disease is poorly understood and recurrent genetic abnormalities have not been systematically characterized. Using targeted exon capture and next-generation sequencing, Dr. Oreofe Odejide and colleagues in the laboratory of Dr. David Weinstock at Dana-Farber Cancer Institute in Boston examined a panel of 219 genes that were identified as mutated in other hematologic malignancies and correlated the findings with demographic and clinical data in 85 cases of AITL (Figure).
The median overall survival for patients was 18 months and was significantly inferior in patients over the age of 70. Mutations were identified in the coding regions of 80 genes, 34 of which were altered in two or more cases. The genetic changes did not differ according to the presence of EBV. Mutations in TET2 were present in 65 patients (76%), and 43 of 65 harbored two or three mutations in that gene. Twenty-eight patients (33%) had alterations in DNMT3A, all of which were seen in the presence of mutant TET2, and 17 patients (20%) had changes in IDH2, of which 15 also harbored TET2 mutations (Figure). Notably, alterations in TET2 and DNMT3A, which function as epigenetic modifiers of DNA, and in IDH2, a mitochondrial enzyme that when mutated produces the presumed oncometabolite 2-hydroxygluterate, are commonly found in myeloid neoplasms, and in contrast to many other lymphoid malignancies, alterations in TP53 were identified in only four cases. Given recent evidence suggesting that mutations in TET2 or DNMT3A may be derived from CD34+ progenitor cells, the investigators examined cellular subsets from a peripheral blood stem cell collection from a patient with AITL in remission whose tumor cells had been shown to harbor a TET2 mutation. That mutation was identified only in the lineage-negative CD34-/ CD38+ fraction of cells and not in the patient’s CD34+ positive cells or in cells with an immunophenotype similar to that of the patient’s AITL cells. The authors used the same experimental design to characterize the origin of the mutant cells in a patient with histology that overlapped AITL and peripheral T-cell lymphoma. In contrast to the prior case in which the mutation arose in a lineage-negative subpopulation, mutations in this case were acquired within lineage-committed progenitors. Additional investigation is needed to understand the origins of AITL.
In Brief
The prognosis of AITL is poor. The optimal management of the disease has not been well defined, and outcomes following anthracycline-based chemotherapy are dismal. Although a small minority of patients will achieve long-term disease control following stem cell transplantation, the vast majority of patients die of progressive disease. The work of Dr. Odejide and colleagues, identifying recurrent and overlapping mutations in TET2, DNMT3A, and IDH2, as well as recent reports demonstrating frequent alternations in RHOA, a small GTPase that regulates diverse biologic processes,1,2 raise the exciting possibility of novel therapeutic approaches in this disease. Studying drugs that work through epigenetic control over transcription, as well as investigating the efficacy of inhibitors of IDH2, may provide critically needed advances in the management of AITL.
References
Competing Interests
Dr. LaCasce indicated no relevant conflicts of interest.