Editor’s note
Data published in this paper were presented in abstract form by Dr. Krönke at the Late-Breaking Abstract Session during the 2013 ASH annual meeting in New Orleans.
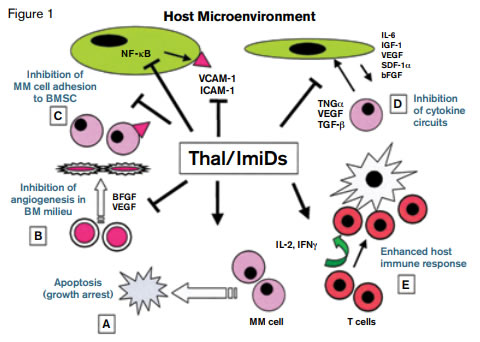
Thalidomide (Thal) and Immunomodulatory Drugs Mechanisms of Action on Multiple Myeloma Cells, Tumor Microenvironment, and Host Immunity. Reprinted with permission © 2014 American Society of Clinical Oncology. All rights reserved. Richardson, P et al: Immunomodulatory analogs of thalidomide: an emerging new therapy in myeloma. J Clin Oncol. Vol. 22 (16), 2004:3212-3214.
Thalidomide (Thal) and Immunomodulatory Drugs Mechanisms of Action on Multiple Myeloma Cells, Tumor Microenvironment, and Host Immunity. Reprinted with permission © 2014 American Society of Clinical Oncology. All rights reserved. Richardson, P et al: Immunomodulatory analogs of thalidomide: an emerging new therapy in myeloma. J Clin Oncol. Vol. 22 (16), 2004:3212-3214.
The publication of thalidomide’s remarkable and unanticipated anti-myeloma activity in 19991 heralded the anti-cancer successes of its more potent analogs, lenalidomide and pomalidomide, over this past decade. The reemergence of these therapeutics, known as immunomodulatory drugs (IMiDs), as effective treatment for myeloma and other hematologic malignancies is a bookend to thalidomide’s tragic beginnings, which are inexorably linked to phocomelic infants born to women who took the drug to mitigate emesis gravidarum. How thalidomide’s teratogenicity interdigitates with its biologic activity in various malignancies has been a focus of intense investigation for more than 20 years. Early illustrations depicting the mechanism of action of thalidomide and lenalidomide highlighted autocrine and paracrine interactions between myeloma cells and stromal cells of the surrounding marrow microenvironment. An array of imaginative artistic techniques were used to propose mechanisms of action that involve anti-angiogenic and immunomodulatory pathways, induction of oxidative stress, and modulation of cytokines, including downregulation of tumor necrosis factor-α and up-regulation of interleukin-2, which stimulates T-cell production (Figure 1).
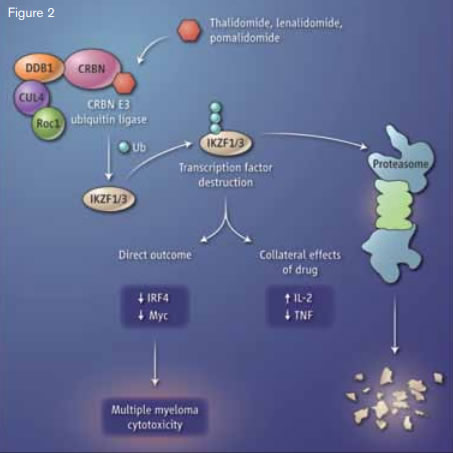
Immune Modulators and Myleoma. The small-molecule drugs thalidomide, lenalidomide, and pomalidomide bind to the protein cereblon (CRBN), which activates the enzymatic activity of the CRBN E3 ubiquitin ligase complex. The transcription factors Ikaros (IKZF1) and Aiolos (IKZF3) are modified with ubiquitin (Ub) molecules, targeting them for proteolysis. This alters the function of T cells and B cells, with a toxic outcome for multiple myeloma cells.From Stewart KA. How thalidomide works against cancer. Science. 2014;343:256-257. Reprinted with permission from AAAS.
Immune Modulators and Myleoma. The small-molecule drugs thalidomide, lenalidomide, and pomalidomide bind to the protein cereblon (CRBN), which activates the enzymatic activity of the CRBN E3 ubiquitin ligase complex. The transcription factors Ikaros (IKZF1) and Aiolos (IKZF3) are modified with ubiquitin (Ub) molecules, targeting them for proteolysis. This alters the function of T cells and B cells, with a toxic outcome for multiple myeloma cells.From Stewart KA. How thalidomide works against cancer. Science. 2014;343:256-257. Reprinted with permission from AAAS.
The first major breakthrough in understanding the mechanism of action of thalidomide and its derivatives occurred when thalidomide was shown to bind to cereblon, a critical component of an E3 ubiquitin ligase complex (Figure 2).2 This report tied the teratogenic effects of thalidomide to inhibition of ubiquitination and toxic accumulation of proteins that led to cell death. In the current reports published in Science, two independent groups have identified targets of lenalidomide that account for its therapeutic effect in multiple myeloma. Dr. Gang Lu and colleagues from the laboratory of Dr. William Kaelin, Dana-Farber Cancer Institute, Boston, MA, and Dr. Jan Krönke and colleagues from the laboratory of Dr. Benjamin Ebert, Brigham and Women’s Hospital, Boston, MA, report that treatment with lenalidomide, and other agents in this class, through interaction with cereblon, do not inhibit, but rather selectively enhance the ubiquitination and degradation of Ikaros (IKZF1) and Aiolos (IKZF3), two members of the Ikaros family of zinc finger transcription factors important in B-cell development. This finding was confirmed through two orthogonal screening approaches.
In the work by Dr. Lu et al., 15,483 different genes were cloned into plasmids containing both firefly and renilla luciferase and transfected into mammalian cells in order to identify genes whose expression was affected by lenalidomide. By comparing the signal from these two luciferases, the investigators were able to detect which of the 15,483 genes were transcribed into proteins and how levels of these proteins were affected by lenalidomide. Expression of both IKZF1 and IKZF3 decreased in the presence of lenalidomide. These findings were subsequently validated in two myeloma cell lines, MM1S and L363.
Using a different approach, Dr. Krönke et al. analyzed the proteome and ubiquitinome of MM1S cells after treatment with lenalidomide. By using stable isotope labeling of amino acids in cell culture (SILAC) and mass spectroscopy, the authors identified a significant lenalidomide-induced decrease in both IKZF1 and IKZF3. The authors then went on to show that treatment with lenalidomide produced a decrease in protein (but not mRNA) levels of IKZF1 and IKZF3 in cells lines and in primary patient samples.
Dr. Lu and colleagues showed the importance of modulating expression of IKZF1 and IKZF3 in cell lines, demonstrating that cytotoxicity was observed only in cells in which IKFZ1 and IKFZ3 expression was inhibited by lenalidomide. Additionally, both groups demonstrate that knockdown of either of these two targets is sufficient to induce cytotoxicity. Notably, Dr. Lu and colleagues observed resistance to lenalidomide-induced cytotoxicity in a number of myeloma cell lines. This unresponsiveness appears to be the result of low-level expression of cereblon by the resistant cells, supporting the concept that the cytotoxic effects of lenalidomide are cereblon-dependent through its action on expression of IKFZ1 and IKZF3 (Figure 2).
A number of other purported mechanisms for lenalidomide’s activity in multiple myeloma have been reported, including down-regulation of expression of interferon regulatory factor 4 (IRF4) and up-regulation of expression of interleukin-2 (IL-2). The authors of both papers tied the decrease in IRF4 to the decrease of IKZF1 and IKZF3 induced by lenalidomide, while Dr. Krönke et al. demonstrated a marked decrease in IKZF1 and IKZF3 protein levels in T cells treated with lenalidomide that was accompanied by a coincident increase in IL-2 expression. Based on these observations, it appears that these previously reported effects of lenalidomide (IRF4 down-regulation and IL-2 up-regulation) are downstream effects of a lenalidomideinduced decrease in IKZF1 and IKZF3 (Figure 2).
In Brief
Selective inhibition of transcription factors, molecules otherwise considered undruggable, represents a novel therapeutic paradigm that has emerged from the story of the relationship between IMiDs, cereblon, and IKZF1/IKZF3. Data derived from these studies also provide a foundation for exploiting these interactions to design new therapeutics and to develop strategies for overcoming resistance to IMiDs. However, important issues remain unexplained. The finding that the activity of IMiDs relies on targeting transcription factors for proteasomal degradation seems incongruous with the highly effective clinical response observed when IMiDs are combined with proteasomal inhibitors in the treatment of patients with myeloma. This paradox, and the contrasting roles of Ikaros transcription factors in different disease contexts (e.g., as tumor suppressors in acute lymphocytic leukemia, and as anti-neoplastic targets in myeloma), require better understanding. Further, while several mechanisms of action have been proposed to account for the therapeutic activity of lenalidomide in the treatment of del (5q) MDS, it is unclear whether targeted ubiquitination and destruction of specific transcription factors underlies the efficacy of lenalidomide in this disease.
References
Competing Interests
Dr. Kurtz and Dr. Gotlib indicated no relevant conflicts of interest.