Update/Commentary
The causes, presentation, and National Cancer Institute–Common Terminology Criteria for Adverse Events (NCI-CTCAE) classification of disability from chemotherapy-induced neuropathy have not changed since the original publication of this article in 2014. Unfortunately, no new proven therapies have been identified.1 Herbal medicines are also ineffective.2 A pilot study of an electrical method (the “Scrambler”) was positive, and phase III trials are being planned.3
Updated References
The Case
The patient is a 54-year-old woman with IgA lambda multiple myeloma, receiving dexamethasone, lenalidomide, and bortezomib therapy. She was healthy before her diagnosis but had mild hypercalcemia and mild renal insufficiency at diagnosis, both of which have resolved with treatment.
She is about to begin her third treatment cycle, but reports painful (pain score of 7/10) numbness and paresthesias in her fingers and feet. The former problem causes clumsiness when using her touch-screen tablet and phone because she cannot accurately feel her fingers touch the screens, and she needs the light on at night because she cannot reliably feel when her feet touch the floor. Her Zubrod (ECOG) performance status is 1. She had similar, but less severe, symptoms at the beginning of her last chemotherapy cycle, but she did not mention them to anyone for fear that treatment would be delayed or discontinued.
During her exam, she was anxious, alert, and oriented. She denied both spine pain and bowel or bladder incontinence. Her exam was unremarkable except for the neurologic examination. She had impaired proprioception, decreased vibration and light touch in her hands to the wrists and feet to the ankles, 4/5 hand grip and finger strength bilaterally, and 4/5 ankle strength. Her ankle deep tendon reflexes (DTRs) were absent (flaccid).
Laboratory studies showed normal potassium, BUN, creatinine, glucose, calcium, and magnesium. B12 concentration and thyroid function studies were normal. Her paraprotein concentration showed a continuing decrease in response to treatment.
The Question
What is your approach to assessment and management of patients with chemotherapyinduced peripheral neuropathy?
My Response
Presentation/Physical Findings
The patient’s presentation is typical of chemotherapy-induced peripheral neuropathy (CIPN) that is characterized by tingling (71%), numbness (58%), and paresthesias or dysesthesias (45%), or spontaneous burning, cramping, or aching pain (40%) that is usually symmetric, begins peripherally and progresses proximally.1 Patients often report allodynia (i.e., pain from something that is usually not painful, like cold temperatures or the touch of clothing), or hyperalgesia (i.e., experiencing more pain than usual from a mildly painful stimulus).
The mechanisms of axonal nerve injury vary by agent and include damage to mitochondria (bortezomib, platinum compounds, vincristine, paclitaxel), glial cells and macrophages (bortezomib, oxaliplatin, vincristine, paclitaxel), vasa vasorum (thalidomide, paclitaxel), endoplasmic reticulum (bortezomib), or microtubules (bortezomib, taxanes, and vinca alkaloids).2,3
CIPN induced by vinca alkaloids causes numbness, not pain, and loss of DTRs, proprioception, and motor impairment, such as foot drop.2 Patients receiving thalidomide, bortezomib, or platinum have a mainly sensory neuropathy, which may include loss of vibration, proprioception, and DTRs.2 Taxanes cause both a sensory neuropathy and proximal weakness.2 Only CIPN induced by thalidomide is irreversible.
Evaluation
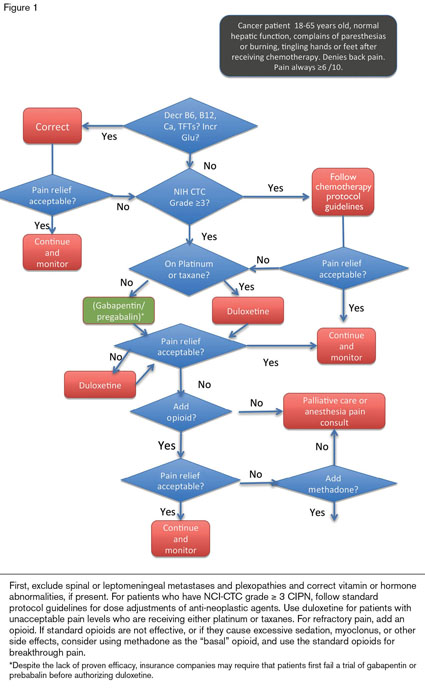
In a patient whose CIPN appears only after chemotherapy, the evaluation should include laboratory testing for B12 deficiency, hypothyroidism, hypocalcemia, hypomagnesemia, and hyperglycemia (Figure 1). Because patients with leptomeningeal carcinomatosis or spinal cord compression can present with these symptoms, an MRI of the spine with contrast is recommended for patients with back pain. If the test is negative, an MRI of the brachial or lumbosacral plexus, depending on the location of the symptoms and clinical suspicion, may be warranted.
Diagnosis of CIPN is clinical, and nerve conduction studies, EMGs, and skin biopsies are not needed for diagnosis or management. Chemotherapy doses are generally adjusted for patients with grade 3 or greater neuropathy based on NCI common terminology criteria (CTC) for adverse events.
NCI-CTC for Neuropathy
Peripheral Sensory Neuropathy
Grade 1: Asymptomatic or loss of deep tendon reflexes or paresthesia
Grade 2: Moderate symptoms limiting instrumental activities of daily living (ADL)
Grade 3: Severe symptoms limiting self-care ADL
Grade 4: Life-threatening consequences; urgent intervention indicated
Grade 5: Death
Peripheral Motor Neuropathy
Grade 1: Asymptomatic clinical or diagnostic observations only; intervention not indicated
Grade 2: Moderate symptoms limiting instrumental ADL
Grade 3: Severe symptoms limiting self-care ADL; assistive device indicated
Grade 4: Life-threatening consequences; urgent intervention indicated
Grade 5: Death
The patient under discussion had grade 2 sensory neuropathy by these criteria, which underestimated her disability and functional losses. The NCI-CTC are not as sensitive to patient-reported symptoms or functional loss as are some other scoring systems, but no consensus has yet been reached on a standard for grading neurotoxicity.4,5 Quality-of-life scales may be more relevant (e.g., the CIPN 20 or FACT/GOG-Ntx).6
Treatment
Dose reduction or drug discontinuation is the only specific therapy for most CIPNs; patients may tolerate higher doses of bortezomib if the drug is given by a subcutaneous injection rather than by IV infusion. Symptomatic therapies for CIPN are limited (Figure 1). Of the adjuvant agents effective for patients with neuropathic pain, only duloxetine (30-60 mg/day) has clearly shown efficacy for the CIPN induced by taxanes and platinum.7 Despite the lack of proven efficacy of other agents in CIPN, insurance companies usually require that patients first fail a trial of gabapentin or pregabalin before authorizing duloxetine. Occupational therapy or physical therapy is recommended for patients with ≥ grade 2 sensory or motor toxicity to improve function and quality of life. Patients should also be assessed for depression, which often accompanies uncontrolled chronic pain.8
While vitamin E, vitamin B6, magnesium, calcium, acetyl-L-carnitine, glutamine, glutathione, n-acetyl cysteine, omega-3 fatty acids, alpha lipoic acid, and cannabinoids each have shown efficacy in non-randomized, non-placebo-controlled trials, none can be recommended yet as standard therapy.1,2,9
Amitriptyline does not prevent or ameliorate chemotherapy-induced neuropathy from vinca alkaloids, platins, or taxanes.1 Venlafaxine, gabapentin, the N-methyl-d-aspartate (NMDA) receptor antagonist dextromethorphan, the oral anesthetics memantine and mexiletine, and low-dose continuous capsaicin treatment are also ineffective.2
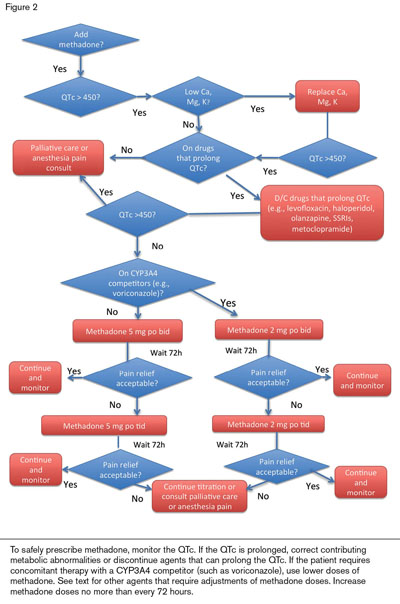
Opioids are recommended for CIPN patients with severe pain.10 Patients will usually require a long-acting agent to provide basal pain relief (e.g., sustained-release morphine or a fentanyl patch) and short-acting opioids for breakthroughs. Methadone (Figure 2) is particularly helpful for basal pain relief. Methadone includes d- and l-isomers that act as opioid-receptor agonists and NMDA receptor antagonists.11 Because NMDA antagonizes the activity of the opiate receptors, blocking NMDA receptors enhances the analgesic effect of externally administered opioids. Methadone also inhibits the reuptake of serotonin and norepinephrine; therefore, it functions as a serotonin-norepinephrine reuptake inhibitor (SNRI).
Methadone is usually given orally bid to tid. Dose adjustment is needed for hepatic failure but not for renal failure. The steady state is not reached until 72 hours after the initial dose or after a dose increase. Therefore, short-acting opioids should be used to control symptoms in the interim. If pain level falls to ≤ 3 within the first 24 to 48 hours of initiating methadone, reduce the dose by half immediately to avoid excessive sedation and respiratory depression that may otherwise occur in the next 24 hours. This delay in reaching steady-state plasma levels, along with potential cardiac toxicity (prolongation of the rate corrected QT [QTc] interval) and its interactions with inducers and inhibitors of the cytochrome P450 system, CYP1A2, CYP3A4, and CYP2D6 make using methadone safely somewhat complex. Palliative care specialists can be helpful in managing initiation or adjustment of methadone doses or for consulting on patients with seemingly refractory pain syndromes.
Patients who, at baseline, have a prolonged QTc interval, or who need high doses of methadone, have developed cardiac arrhythmias (torsades de pointes; polymorphic ventricular tachycardia). Fatalities have been reported but were associated with doses of > 600 mg per day. Common drugs used in hematology patients that also prolong the QTc include the quinolone antibiotics (especially levofloxacin), typical and atypical antipsychotics (such as haloperidol and olanzapine), selective serotonin reuptake inhibitors (SSRIs) (but not SNRIs), and metoclopramide.
Methadone’s drug interactions are listed on websites including www.drugs.comwww.drugs.com or www.qtdrug.orgwww.qtdrug.org. Fluconazole, voriconazole, fluoxetine, and fluvoxamine raise methadone levels. SSRIs may raise methadone levels in CYP2D6 rapid metabolizers but SNRIs (e.g., venlafaxine) do not. Additionally, grapefruit juice and acute alcohol ingestion can increase methadone levels. Drug levels of desipramine (or other tricyclic antidepressants) and zidovudine increase when methadone is added. Antiretrovirals, phenobarbital, carbamazepine, phenytoin, rifampin, somatostatin, spironolactone, and risperidone lower the levels of methadone and have precipitated withdrawal symptoms.
Conclusion
The patient continued therapy, but bortezomib delivery was switched from IV infusion to SQ injection. Her pain symptoms fell to tolerable levels on 2 mg tid of methadone such that she was able to work, and her functional level was further enhanced by participation in an occupational therapy program.
CIPN can be a disabling complication of chemotherapy. Patient education that encourages reporting relevant symptoms, chemotherapy dose adjustment, occupational therapy, physical therapy, and symptomatic therapy using duloxetine and opioids, including methadone, can often help patients maintain or regain function and minimize pain and disability.
References
Author notes
The update/commentary section was added in 2016 when this article was included in the Ask the Hematologist Compendium 2010-2015Ask the Hematologist Compendium.
Competing Interests
Dr. Abrahm receives royalties from her book, A Physician’s Guide to Pain and Symptom management in Cancer Patients, 2nd edition, 2005, from Johns Hopkins University Press. She is the paid section editor for UpToDate on the topic of pain in palliative care patients. She also receives expense reimbursement and honoraria from Knowledge to Practice for her lectures on palliative care.