Cell-to-cell communication is a fundamental component of both normal physiology and pathophysiology, including tumorigenesis. Among the mechanisms by which cells communicate is the generation and processing of exosomes. Exosomes are nanometer-sized endocytic vesicles that are released into the extracellular compartment by many types of cells. The content of exosomes includes proteins, lipids, mRNA, and microRNA. Examples of exosome-modified processes include cellular immunity, response to infection, and tumorigenesis. For example, antigen-driven, T-cell-derived exosomes containing microRNA transfer unidirectionally to antigen-presenting cells where the transferred microRNA modulates gene expression in recipient cells.1 Compelling evidence supports a role for exosomes in infectious disease pathophysiology. Exosomes produced by eukaryotic pathogens such as Cryptococcus neoformans or by host cells infected with intracellular pathogens may either facilitate or limit the infection based on the nature of the infectious organism and the cell type that is targeted by the exosome.2 The function of exosomes in tumor progression can be explained, at least in part, by the capacity of tumor cell-derived exosomes to modulate and mold the host microenvironment and thereby produce conditions that favor proliferation of neoplastic cell population.3-6 Exosome-mediated processes have been implicated in the pathobiology of both solid tumors and hematologic malignancies. In the case of blood cancers, a pathogenic role for exosomes has been demonstrated for multiple myeloma (MM) and leukemias (Figure).
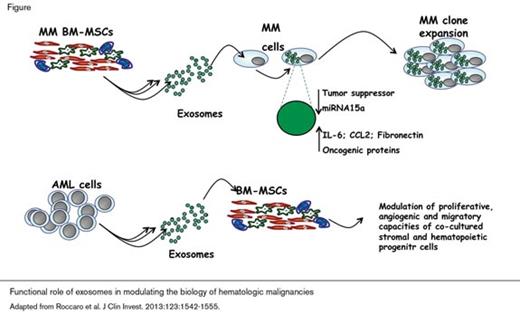
The capacity of the bone marrow mesenchymal stromal cell (BM-MSC) compartment to generate exosomes has been recently characterized under both normal and pathologic conditions. In the case of plasma cell dyscrasias, MM-BM-MSC-derived exosomes were shown to transfer their microRNA and protein content to the malignant plasma cells, thereby enhancing myeloma tumor growth and increasing dissemination of the malignant cells in vivo (Figure).7 Characterization of BM-MSC-derived exosomes showed that the microRNA and protein content was different for exosomes derived from MM-BM-MSCs compared with the content of exosomes derived from normal BM-MSCs. Specifically, exosomal-miRNA-15a levels were significantly lower in MM compared with the levels contained in exosomes derived from normal BM-MSCs, suggesting that exosomal miRNA-15a functions as a tumor suppressor. Additionally, the protein content of exosomes isolated from MM BM-MSCs was found to be enriched for oncogenic proteins, cytokines, and kinases, including IL-6, CCL2, and fibronectin (Figure).7 Together, the results of these experiments suggest that BM-MSCs support plasma cell tumorigenesis not only through paracrine mechanisms involving secretion of growth factors,8-10 but also through direct transfer of microRNAs and proteins from exosomes derived from MM BM-MSCs. Thus, MM BM-MSCs create a favorable niche for expansion of the neoplastic clone (Figure).
Recent studies have shown that primary acute myeloid leukemia (AML) cells release exosomes enriched for both coding and noncoding RNAs. In this case, AML-derived exosomes deliver their content to bystander cells, including BM-MSCs, consequently modulating proliferative, angiogenic, and migratory properties of co-cultured stromal and hematopoietic progenitor cell lines (Figure).11 Analysis of exosome content may also have potential as a biomarker of disease, as mRNAs that encode proteins including NPM1, FLT3, CXCR4, MMP9, and IGF-1R were detected in exosomes isolated from patient-derived AML blasts and leukemic cell lines. Characterization of these exosomes showed that the mRNA content reflected the FLT3 and NPM1 allelic diversity of the cell population from which they were isolated. Thus, analysis of exosome content may provide actionable clinical information.
Exosome formation by tumor cells may also affect response to therapy. For example, in patients with B-cell lymphoma, tumor-derived exosomes may limit the efficacy of anti-CD20 immunotherapy. In this case, antigen binding to the B-cell receptor stimulates release of exosomes expressing CD20 into the extracellular space where they bind anti-CD20, shielding tumor cells from antibody attack.12,13 Both in vitro and in vivo studies demonstrated that rituximab bound to the CD20-expressing exosomes activated complement, leading to complement consumption that reduced the antitumor efficacy of anti-CD20-driven complement-dependent cytolysis. In those studies, exosome biogenesis was found to be modulated by the lysosome-related, organelleassociated ATP-binding cassette transporter A3 (ABCA3).13 Pharmacologic blockade of ABCA3 using the cyclooxygenase type-2 inhibitor indomethacin inhibited exosome release, thereby reducing the sump effect of CD20-bearing exosomes and enhancing the anti-tumor efficacy of rituximab.
Together, the above studies provide insights into the mechanisms by which exosomes contribute to the pathobiology of hematologic malignancies. In MM, BM-MSCderived exosomes transfer microRNA and protein to the malignant plasma cells thereby enhancing myeloma tumor growth and increasing dissemination of the malignant cells (Figure). AML-derived exosomes can re-program the bone marrow niche to support tumorigenesis (Figure), and B-cellderived exosomes uniquely contribute to drug resistance by diverting immunotherapy from its intended target. Continued investigation of the properties of exosomes is warranted both to understand more completely the role of exosomes in the pathophysiology of hematologic malignancies and to identify novel approaches to therapy.
References
Competing Interests
Dr. Roccaro, Dr. Manier, and Dr. Ghobrial indicated no relevant conflicts of interest.