Liver Transduction in the Presence and Absence of Factor X. Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine. 2013;19:455, copyright 2013.
Liver Transduction in the Presence and Absence of Factor X. Reprinted by permission from Macmillan Publishers Ltd: Nature Medicine. 2013;19:455, copyright 2013.
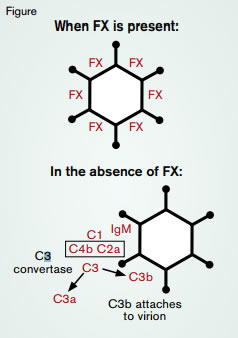
Adenoviruses are the gene therapy vectors used most frequently in humans,1 and adenovirus type 5 (Ad5) is the most commonly used of the adenoviral vectors. Many viral vectors are recognized by natural antibodies (i.e., antibodies that develop in the absence of prior antigen exposure). Such natural IgM antibodies limit vector utility because IgM is a pentameric immunoglobulin that efficiently activates the classical pathway of complement, thereby neutralizing the vector as a consequence of C3b opsonization (Figure). Notably, however, Ad5 is not neutralized by this natural antibody/complement mechanism, which makes it a more effective vector.
Zhili Xu and colleagues, in the laboratory of Andrew Byrnes at the Center for Biologics Evaluation and Research at the U.S. Food and Drug Administration, now find that factor X (FX) enhances liver transduction by Ad5 vectors by protecting Ad5 from attack by the classical complement pathway (Figure). This finding is congruent with reports that adenoviruses bind coagulation factors. In particular, high-affinity binding of FX to Ad5 is required for efficient transduction of hepatocytes.2 In support of this observation, the authors found that the Ad5 mutant vectors, Ad5BAP and AdHVR7, which weakly bind FX, inefficiently transduce mouse livers. Xu et al. also observed that transduction of liver in vivo by a wild-type Ad5 vector was inhibited in normal mice treated either with warfarin (which reduces the levels of vitamin K-dependent proteins, including FX) or with the FX-binding protein, X-bp. However, liver transduction was normal in warfarin- or X-bp-treated Rag1−/− mice, which lack mature T and B lymphocytes and therefore cannot generate antibodies. Transplantation of normal mouse bone marrow into Rag1−/− mice restored the requirement of FX for efficient transduction. This result set the stage for experiments designed to identify the cell that limits transduction efficiency in the absence of FX. Warfarin treatment had no effect on liver transduction in SCID mice, which lack B and T cells, or in Igh-6−/− or JHD mice, both of which lack B cells. In contrast, warfarin treatment inhibited liver transduction in nude mice, which lack T cells, but not B cells. Thus, B cells are necessary for the immunologic neutralization of Ad5 in the absence of FX. Further, warfarin did not inhibit transduction in membrane immunoglobulin transgenic (mIg Tg) mice, which have B cells that express membrane-bound IgM but do not secrete IgM. However, warfarin inhibited liver transduction by Ad5 of JHD mice treated with purified IgM. Transduction was not inhibited by warfarin in C1q-deficient and C4-deficient mice (i.e., mice that lack the capacity to generate C3b opsonization through classical pathway activation). Collectively, these results support the hypothesis that activation of the classical pathway by natural anti-viral IgM antibodies is the major mechanism involved in suppressing liver transduction by Ad5 in FXdeficient mice.
In vitro experiments were performed to further characterize the interaction of natural antibodies, complement, and FX during Ad5 vector transduction. Normal mouse serum, which was used as a source of complement, did not inhibit the infectivity of a normal Ad5 vector. However, it markedly neutralized the non-FX-binding mutant AdHVR7. Purified IgM added to IgM-deficient serum, but not IgM-deficient serum alone, neutralized AdHVR7. Furthermore, the normal Ad5 vector was neutralized by mouse serum when FX binding was blocked by X-bp.
Western blotting experiments showed that, in the absence of functional FX, C3b bound covalently to Ad5 virions (Figure). In these experiments, purified normal Ad5 or AdHVR7 virions were incubated with serum-containing complement and isolated by density centrifugation. Following SDS-PAGE, which dissociates non-covalent complexes, covalently bound C3b was detected bound to AdHVR7 proteins. However, covalent binding of C3b to normal Ad5 proteins was not observed. Additionally, incubation of AdHVR7 with mouse plasma resulted in the conversion of C3 into C3a and C3b (a finding indicative of complement activation), but C3 conversion was not observed in samples containing the normal Ad5 vector unless X-bp was added to inhibit FX binding (Figure). Together, these results demonstrate that FX protects virions from C3b opsonization mediated by IgM antibody-induced activation of the classical pathway of complement (Figure).
In Brief
The authors have identified a defense mechanism in which FX enhances virus survival by inhibiting attack by natural antibodies and complement. This observation has practical implications for the use of adenoviral vectors in gene therapy. Specifically, defense against complement-inactivating mechanisms could be engineered into novel adenoviral vectors.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.