Pidilizumab Blocks the Inhibitory Programmed Death-1 (PD-1) Receptor, Which is Present on Activated T Cells, Activated B Cells, Natural Killer Cells, and Myeloid Cells
Pidilizumab Blocks the Inhibitory Programmed Death-1 (PD-1) Receptor, Which is Present on Activated T Cells, Activated B Cells, Natural Killer Cells, and Myeloid Cells
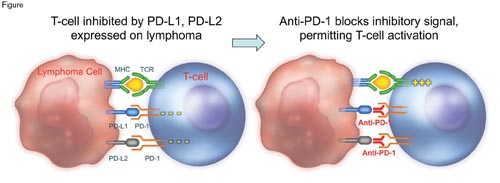
Lymphomas should be among the most immunogenic of malignancies because the neoplastic cells express both HLA class I and II antigens at high density. The graft-versus-lymphoma effect seen following allogeneic stem cell transplantation supports this impression. Although attempts to produce a similar effect in the autologous transplant setting have been largely unsuccessful, the development of antibodies with the capacity to disinhibit the cellular immune response has generated new interest in immunotherapy for lymphoproliferative disorders. Two recent studies examining pidilizumab give an indication of the potential of this therapeutic approach. Pidilizumab blocks the inhibitory programmed death-1 (PD-1) receptor, which is present on activated T cells, activated B cells, natural killer cells, and myeloid cells (Figure). On T cells in particular, PD-1 is a marker of cellular exhaustion as it conveys signals that inhibit T-cell proliferation and enhance apoptosis, consequently functioning as a negative regulator of the cellular immune response (Figure). Specific ligands (PD-L1 and PD-L2) for PD-1 are expressed by a variety of normal cells and by many tumor cell types, and compelling data indicate that activation of the PD-1 signaling system by PD-L1 and PD-L2 attenuates T-cell activity, thereby allowing tumor cells to escape immune surveillance (Figure). The good news is that the negative effects of the PD-1 signaling system can be limited pharmacologically both by antibodies that block PD-1 and by antibodies that block its activating ligands, PD-L1 and PD-L2 (Figure).
To investigate the clinical efficacy of anti-PD-1 therapy, an international group of investigators led by Leo I. Gordon and colleagues from Northwestern University Feinberg School of Medicine in Chicago administered three doses of pidilizumab at six-week intervals to 66 patients with diffuse large B-cell lymphoma (DLBCL) following treatment with high-dose therapy and autologous stem cell rescue. Although this was a single-arm study with no control group, the finding that 72 percent of patients were free of disease progression 16 months after completing the regimen was promising, especially given that outcomes were the same for the 24 high-risk patients who had a positive FDG-PET at the time of enrollment into the study (i.e., prior to treatment with high-dose chemotherapy followed by autologous stem cell rescue.) Among patients with residual disease visible on CT scans at the time antibody treatment was started, a little over half had an objective response. Although an argument could be made that this outcome might be in part attributable to the ongoing effect of the high-dose therapy, the high response rate for this poor-risk subpopulation is nonetheless encouraging.
A second single-arm study that focused on recurrent follicular lymphoma was led by Sattva S. Neelapu from MD Anderson Cancer Center in Houston. The target group was patients with relapsed disease who had previously demonstrated rituximab sensitivity. Up to 12 doses of pidilizumab were given to patients before and after four weeks of rituximab. The overall response rate was 66 percent (19 out of 29 patients), and a median progression-free survival (PFS) of 19 months was projected. In both studies, the investigators made attempts to determine the mechanisms of action of pidilizumab and to define markers of response. In the diffuse large B-cell transplant study, flow cytometry was used to quantify the number of both circulating helper T cells expressing PD-L1 and circulating peripheral and central memory T cells following pidilizumab therapy. The results of this analysis suggested an on-target effect of the anti-PD-1 reagent. In the follicular lymphoma study, the density of PD-L1 on T cells and monocytes was predictive of response to the antibody combination, and, using gene-expression profiling, the investigators observed that the presence of transcripts reflecting an activated T-cell phenotype prior to therapy correlated with improved PFS.
Concerns have been expressed about the potential toxicity that might result from disinhibited cellular immunity, but the findings in these two studies were reassuring. In the DLBCL study, the most common grade 3-4 toxicity was myelosuppression, with one case of fatal disseminated Herpes zoster infection reported, while in the follicular lymphoma study, no grade 3-4 toxicity was reported. This toxicity profile is more benign than that observed in studies using anti-CTLA-4 that unblocks earlier T-cell activation, as treatment with this antibody can be complicated by severe autoimmune disease. It has been suggested that anti-PD-1 acts to release suppression of the T-cell response in peripheral tissues, accounting for the more favorable toxicity profile compared with treatment with anti-CTLA-4 that results in unrestricted T-cell activation.
In Brief
Modulation of the cellular immune response using checkpoint-blocking antibodies such as anti-PD-1 and anti-CTLA-4 has recently been identified as a potentially effective therapeutic strategy in the treatment of immunogenic tumor types such as melanoma. The results of the above two trials further reinforce the concept that lymphoma is immunogenic to the host’s autologous cellular immune system, building upon previous studies that used therapeutic approaches such as systemic cytokines, vaccines, and toll-like receptor agonists to stimulate the immune response. While promising, this type of immune modulation is in its infancy as a therapeutic modality with many outstanding issues and unanswered questions such as how to predict who will respond, the precise mechanism of action by which the antibodies disinhibit the cellular immune response, and how to avoid autoimmune-mediated toxicity. The results of studies using anti-PD-1 are encouraging, but the findings need to be confirmed in randomized trials. If the data hold up to rigorous investigation, further studies using anti-PD-1 antibodies either singly or in combination with chemotherapy, targeted kinase inhibitors, immunomodulatory drugs, radiation, other antibodies, and perhaps also vaccines will likely ensue. Activation of host anti-tumor immunity represents an exciting new direction for lymphoma therapy that will certainly be the subject of intense basic and clinical investigation.
Competing Interests
Dr. Johnson indicated no relevant conflicts of interest.