The Question
When do you recommend light transmission aggregometry as part of the evaluation of a patient with a suspected bleeding disorder?
My Response
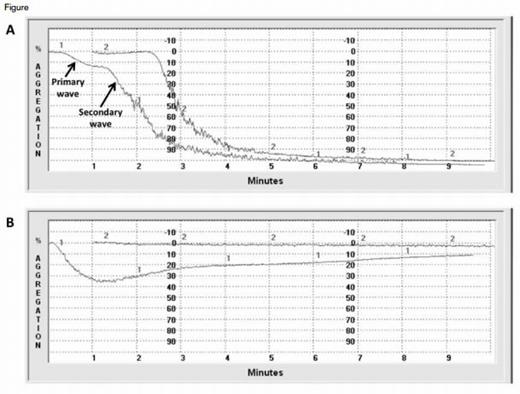
Representative Normal and Abnormal LTA Tracings. Figure A shows normal responses to epinephrine 1.0 μM (curve 1) and collagen 2.0 mg/mL (curve 2). At critical concentrations, weak agonists such as epinephrine and ADP induce a biphasic response. The primary wave of aggregation reflects response to the addition of exogenous agonist. When normal platelets are activated, they secrete an endogenous pool of agonists. The secondary wave represents aggregation in response to this endogenous pool. Figure B is a tracing from a patient with epistaxis and menorrhagia. The response to ADP 5.0 μM (curve 1) is attenuated. A primary wave of aggregation is followed by deaggregation and an absent secondary wave of aggregation. There is no response to epinephrine 5.0 μM (curve 2). The patient’s platelets also demonstrated abnormal ATP secretion in response to ADP and epinephrine. Electron microscopy of the patient’s platelets revealed an absence of dense granules consistent with a diagnosis of dense granule storage pool disorder.
Representative Normal and Abnormal LTA Tracings. Figure A shows normal responses to epinephrine 1.0 μM (curve 1) and collagen 2.0 mg/mL (curve 2). At critical concentrations, weak agonists such as epinephrine and ADP induce a biphasic response. The primary wave of aggregation reflects response to the addition of exogenous agonist. When normal platelets are activated, they secrete an endogenous pool of agonists. The secondary wave represents aggregation in response to this endogenous pool. Figure B is a tracing from a patient with epistaxis and menorrhagia. The response to ADP 5.0 μM (curve 1) is attenuated. A primary wave of aggregation is followed by deaggregation and an absent secondary wave of aggregation. There is no response to epinephrine 5.0 μM (curve 2). The patient’s platelets also demonstrated abnormal ATP secretion in response to ADP and epinephrine. Electron microscopy of the patient’s platelets revealed an absence of dense granules consistent with a diagnosis of dense granule storage pool disorder.
Light transmission aggregometry (LTA) remains the reference method for measurement of platelet function in patients with suspected platelet function disorders (PFDs). Remarkably, the core principles on which LTA is based have not changed since it was first described by O’Brien and Born more than 50 years ago.1,2 Platelet-rich plasma is stirred in a cuvette that is placed between a light source and a photocell. The plasma is cloudy due to suspension of platelets and allows relatively little light to pass through. Upon the addition of an agonist, platelets aggregate, and the sample becomes clearer, permitting greater light transmission. Transmission of light is detected by the photocell and recorded as a function of time (Figure). An enhancement of modern LTA is the capacity to simultaneously monitor ATP secretion from dense granules using a luciferinluciferase reagent.3
When do I recommend LTA?
Rare PFDs such as Glanzmann thrombasthenia (GT) and Bernard–Soulier syndrome (BSS) are usually diagnosed early in life because of the severity of the bleeding phenotype. In syndromic PFDs, the presence of associated features (e.g., oculocutaneous albinism in Hermansky–Pudlak syndrome) may facilitate diagnosis. Far more common, however, are mild PFDs without associated syndromic features. Even with a detailed bleeding history, these disorders may be difficult to distinguish from normal variation due to the high frequency of mucocutaneous bleeding symptoms in the general population. In one study of healthy adults, epistaxis, easy bruising, and prolonged bleeding after tooth extraction were reported in 25 percent, 18 percent, and 18 percent of subjects, respectively; and 47 percent of menstruant women reported heavy menses.4
A bleeding history may be taken using either a conventional approach or a standardized bleeding assessment tool (BAT). Published BATs are effective in discriminating patients with bleeding disorders from healthy controls, but they have not proven effective in predicting the presence of PFDs among patients referred for a suspected bleeding disorder. A recent study investigated the utility of the International Society on Thrombosis and Haemostasis (ISTH)-BAT for this purpose. Twenty-one healthy controls and 79 patients with a suspected bleeding disorder were enrolled. A normal basic hemostatic work-up (platelet count, PT, APTT, fibrinogen, and von Willebrand panel) was required for study entry. The median ISTH-BAT score was significantly greater in patients than in controls (12 vs. 0). Among the patients who had a suspected bleeding disorder, however, there was no difference in score between the 41 who were diagnosed with a PFD by LTA and the 38 with a normal LTA (11 vs. 12).5
In my practice, I employ a conventional approach to the bleeding history. I query patients about spontaneous, mucocutaneous bleeding symptoms (location, frequency, severity, treatment); bleeding with hemostatic challenges (e.g., surgery, trauma, childbirth, menstruation); and family history of bleeding. I also ask about prescription and over-the-counter medications (e.g., anti-platelet agents) and comorbidities (e.g., liver disease, uremia) that could promote bleeding. I use data from the history to estimate the pretest probability of a PFD. I recommend LTA when I judge the pretest probability to be intermediate or high (i.e., >5-10%). My approach is likely to be highly operator-dependent and result in over-testing (only about half of the patients I refer for LTA have an identifiable defect). Although BATs hold promise for ameliorating these deficiencies, I have opted not to use BATs in my practice until they are shown to improve diagnostic accuracy compared with conventional approaches to assessment of bleeding.
How is LTA interpreted?
A panel of seven platelet agonists – ADP, epinephrine, collagen, thrombin receptor-activating peptide, the thromboxane A2 mimetic U46619, arachidonic acid, and ristocetin – is recommended by an international consensus panel.6 Aggregation tracings (Figure) are evaluated with respect to a number of parameters including shape change; length of lag phase; slope of aggregation; presence of a secondary wave of aggregation produced by weak agonists, such as epinephrine; maximal percent aggregation; and presence of deaggregation.
Table. Characteristic LTA Patterns of Selected Platelet Function Disorders
| Disorder . | Aggregation Response . | Other Features . | |||||
|---|---|---|---|---|---|---|---|
| Primary ADP . | Secondary ADP . | Epinephrine . | AA . | Collagen . | Ristocetin . | ||
| Inherited disorders | |||||||
| GT | Absent | Absent | Absent | Absent | Absent | Normal | |
| BSS | Normal | Normal | Normal | Normal | Normal | Absent | Macrothrombocytopenia |
| Dense granule SPD | Normal | Decreased or Absent | Variable | Normal | Normal | Normal | Deficiency of dense granules seen with platelet electron microscopy. May be syndromic or occur in isolation. |
| Acquired disorders | |||||||
| Aspirin | Normal | Decreased or Absent | Decreased or Absent | Absent | Decreased or Absent | Normal | Inherited aspirin-like defects in AA metabolism show a similar pattern |
| P2Y12 inhibitors (e.g., clopidogrel) | Decreased or absent | Absent | Normal | Normal | Normal | Normal | |
| Glycoprotein 11b/111a inhibitors | Absent | Absent | Absent | Absent | Absent | Normal | |
| Uremia | Normal | Decreased | Variable | Decreased | Normal or Decreased | Normal | |
| Disorder . | Aggregation Response . | Other Features . | |||||
|---|---|---|---|---|---|---|---|
| Primary ADP . | Secondary ADP . | Epinephrine . | AA . | Collagen . | Ristocetin . | ||
| Inherited disorders | |||||||
| GT | Absent | Absent | Absent | Absent | Absent | Normal | |
| BSS | Normal | Normal | Normal | Normal | Normal | Absent | Macrothrombocytopenia |
| Dense granule SPD | Normal | Decreased or Absent | Variable | Normal | Normal | Normal | Deficiency of dense granules seen with platelet electron microscopy. May be syndromic or occur in isolation. |
| Acquired disorders | |||||||
| Aspirin | Normal | Decreased or Absent | Decreased or Absent | Absent | Decreased or Absent | Normal | Inherited aspirin-like defects in AA metabolism show a similar pattern |
| P2Y12 inhibitors (e.g., clopidogrel) | Decreased or absent | Absent | Normal | Normal | Normal | Normal | |
| Glycoprotein 11b/111a inhibitors | Absent | Absent | Absent | Absent | Absent | Normal | |
| Uremia | Normal | Decreased | Variable | Decreased | Normal or Decreased | Normal | |
GT=Glanzmann thrombasthenia; BSS=Bernard-Soulier syndrome; SPD=storage pool disorder; ADP=adenosine diphosphate; AA=arachidonic acid
The pattern of aggregation and/or secretion defects observed with the agonist panel facilitates identification of a variety of hereditary and acquired PFDs (Table) and also helps to localize lesions to specific pathways. Classic examples include GT (caused by mutations in integrin αIIbβ3) and BSS (caused by mutations in the platelet glycoprotein Ib-IX-V complex), both of which have characteristic LTA signatures (absent aggregation to all agonists except ristocetin and absent ristocetin-induced agglutination, respectively).
In practice, LTA abnormalities often do not conform to one of the textbook patterns shown in the Table. Many of these “non-specific” abnormalities are of uncertain clinical significance and molecular pathogenesis. However, critical new insights are emerging from genotype–phenotype correlation analyses such as the United Kingdom Genotyping and Platelet Phenotyping (GAPP) study.7 GAPP is a multicenter study of patients with clinically suspected inherited PFDs. Results from the first 111 patients were recently reported.8 A defect by LTA was detected in 64 patients (58%). Most defects (84%) involved Gi (i.e., ADP and epinephrine) receptor signaling, thromboxane A2 receptor signaling, or dense granule secretion. Targeted genotyping subsequently identified novel mutations in the P2Y12 ADP receptor, the thromboxane A2 receptor, and FLI1 and RUNX1, genes encoding transcription factors that participate in megakaryopoiesis and dense granule secretion.8,9
Several limitations must be borne in mind in the interpretation of LTAs. First, some LTA “abnormalities” are observed in a small percentage of healthy volunteers. Clinical correlation is required, but not always conclusive, in distinguishing disease from normal variation. LTA cannot assess platelet function under flow conditions and may be influenced by a variety of preanalytic, analytic, and biologic variables.
Which variables can confound LTA?
Many drugs affect platelet function in vitro. Drugs with reversible effects on platelets (e.g., NSAIDs) should be held for at least three days and drugs with irreversible effects (e.g., aspirin, clopidogrel) for at least 10 days prior to testing. An international consensus panel recommends a short rest period and avoidance of smoking and caffeine prior to blood collection to mitigate the effects of epinephrine release.6 Because chylomicrons in plasma can interfere with LTA, patients should be counseled to avoid fatty meals shortly before testing. Laboratories must adhere to meticulous specifications for sample collection and processing to avoid in vitro platelet activation. The platelet count in platelet-rich plasma should be measured. Results may be inaccurate when the platelet count is < 150 × 109/L. Studies should be completed within four hours after blood collection.6
Conclusion
As they have for decades, a detailed bleeding history and LTA form the backbone of the diagnostic evaluation of patients with suspected PFDs. These approaches are not without shortcomings. The bleeding history has relatively poor specificity because of the frequency of mucocutaneous bleeding symptoms in the general population. LTA is labor-intensive, requires considerable expertise and a fresh blood sample, is not well-standardized, and shows overlap between normal variation and pathology across certain parameters. Continued development of BATs and improved methods for measuring platelet function are needed to overcome these limitations. Genotype–phenotype correlation studies are elucidating novel defects, are providing new insights into platelet function in health and disease, and may ultimately pave the way for more exacting approaches to diagnosis.
References
Author notes
In 2016, this article was included in the Ask the Hematologist Compendium 2010-2015Ask the Hematologist Compendium. At that time, the author indicated that there had been no update regarding the content of this article since the original publication date in 2014.
Competing Interests
Dr. Cuker indicated no relevant conflicts of interest.