Mechanisms Whereby Malignant B Cells Modulate the Function of Intratumoral T Cells.
Mechanisms Whereby Malignant B Cells Modulate the Function of Intratumoral T Cells.
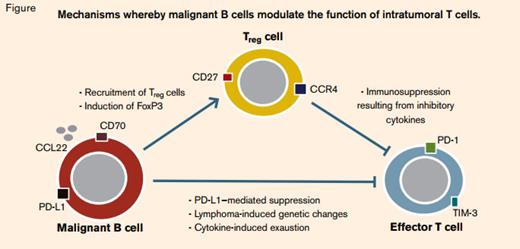
There is a good deal of circumstantial evidence to suggest that follicular lymphoma is potentially immunogenic, including occasional spontaneous regressions and its strikingly long latency. Supporting information is derived from studies of gene-expression profiling showing that the signature of infiltrating T cells and macrophages appears to correlate with clinical outcomes.1 However, it is not clear how these relationships work, and the published literature can be confusing. The situation is made worse by the confounding effects of treatment; if patients receive chemotherapy, the presence of an inflammatory infiltrate seems unfavorable, whereas for those receiving an anti-CD20 antibody it may be helpful. Similar to other tumors, it seems likely that follicular lymphoma can exert a locally immunosuppressive effect (Figure), but unlike epithelial cancers, nodal lymphoma arises in a tissue already consisting of a mixed infiltrate of T cells with which the neoplastic B cells normally have multiple cognate interactions (Figure). This study from Kiaii and colleagues at Saint Bartholomew’s Hospital in London provides some interesting mechanistic data about the differences between normal and malignant lymph nodes, and the interplay of B and T cells within them.
Gene-expression profiles in purified tumor-infiltrating T cells (TILs) taken from lymphoma biopsies were compared with T cells taken from reactive tonsils, and a number of genes were identified as deregulated in both CD4+ and CD8+ cells. For certain specific genes (PMCH, ETV1, CD200, and NAMPT), the findings from the microarray analysis were confirmed by RT-PCR and by immunohistochemistry. The phenotypic changes in TILs could be reproduced in normal T cells by co-culture with malignant lymphoma B cells, although some genes (PMCH, ETV1) were upregulated in transwell cultures that keep the B and T cells physically separate, while others (CAV1) required direct cell-cell contact. One of the most strongly down-regulated genes in lymphoma TILs was ACTN1, which prompted the group to examine the changes in B-cell motility in vitro. They showed that co-culture of healthy T cells with lymphoma B cells significantly reduced their motility, suggesting that greater motility of follicular lymphoma cells as a result of inhibition of T-cell-derived production of alpha-actinin-1 may contribute to the neoplastic phenotype. Finally, Kiaii and colleagues examined the prognostic significance of three of the upregulated T-cell genes and showed that levels of PMCH, ETV1, and NAMPT measured by immunohistochemistry correlated with overall survival and time to transformation, although the relationships were complex, depending on the specific location of the T cells within the node (interfollicular or intrafollicular). It was notable that the genes identified in this study were distinct from those determined to be prognostic in previous gene-expression array experiments that used whole-nodal mRNA preparations.
In Brief
Normal nodal physiology involves a dense set of interactions between B and T cells, and even malignant B cells from follicular lymphoma require additional signals from their microenvironment to maintain them in vitro.2 It is clear from this study and others that the signals go strongly in the other direction too, with malignant B cells having the capacity to produce important phenotypic changes in TILs, specifically immunosuppressive effects. Although our understanding of this process is still in the early stages, characterization of the immune regulatory properties of tumor cells is becoming increasingly important as immunomodulating approaches to treatment such as induction of innate immune responses by CpG, T-cell modulation with anti-PD-1 or anti-PDL-1 antibodies, or adoptive cellular therapy with modified chimeric antigen-receptor-bearing T cells enter the clinical arena. The striking clinical results seen with the combination of rituximab and lenalidomide support the idea that future therapeutic approaches are likely to involve combination immunotherapy.3 Elucidating the mechanisms that underlie lymphoma’s immune escape strategies will become increasingly relevant as development of novel therapeutics that target their reversal is now feasible.
References
Competing Interests
Dr. Johnson indicated no relevant conflicts of interest.