Dr. Gotlib is a contributing author of this study. He receives funding for administration of clinical trials from Incyte, manufacturer of ruxolitinib, and serves on an Incyte advisory board.
Constitutive activation of tyrosine kinases (TKs) and related signaling pathways has become the pathogenetic sine qua non of myeloproliferative neoplasms (MPNs). This theme is exemplified by the BCR-ABL fusion oncogene in chronic myeloid leukemia; the JAK2 V617F mutation in polycythemia vera, essential thrombocythemia, and primary myelofibrosis; KIT D816V in systemic mastocytosis; and rearranged PDGFRA/B and FGFR1 in myeloid (and sometimes lymphoid) neoplasms associated with eosinophilia. CML and eosinophilic neoplasms with activated PDGFRA/B are emblematic of the successful application of small molecule inhibitors (e.g., imatinib), especially in the earlier phase of illness when a singular genetic lesion is the predominant driver of the disease. In intermediate- to high-risk myelofibrosis where increasing genetic complexity supervenes, the benefits of JAK inhibitors are more palliative in nature and are typically characterized by mitigation of splenomegaly and disease symptoms without hematologic or molecular remissions. The basis for these more modest responses also partly relates to the fact that JAK inhibitors are not specific to mutant JAK2, as they also inhibit the wild-type protein.
Chronic neutrophilic leukemia (CNL) is a very rare MPN that is characterized by leukocytosis (>25,000/mm3 ) and a differential consisting of > 80 percent neutrophils and band forms. Exclusionary criteria include the finding of BCR-ABL or PDGFRA/B and reactive causes of neutrophilia. Non-specific cytogenetic abnormalities have been described in CNL, and mutations such as JAK2 V617F have been described in a few cases. CNL may show overlapping clinical and laboratory features with MDS/MPNs such as atypical (BCR-ABL negative) CML and chronic myelomonocytic leukemia (CMML), but the presence of dysplasia or prominent myeloid immaturity or monocytosis mitigates against the diagnosis. The prognosis of CNL is generally poor, with death usually resulting from bleeding, infection, or evolution to acute myeloid leukemia. Conventional chemotherapeutics (e.g., hydroxyurea) may produce temporary disease control, but they do not modify the natural history of the illness. Given its rarity and the absence of a disease-defining therapeutic target, CNL has remained an esoteric myeloid neoplasm compared with its more visible MPN cousins.
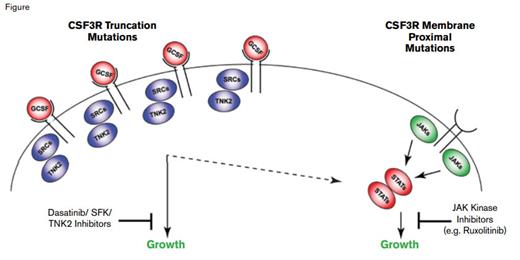
Jeffrey Tyner and Julia Maxson and colleagues from the Oregon Health and Sciences University led a multi-institutional study to identify unknown molecular targets in primary leukemia samples, with a focus on CNL and aCML given the paucity of mutation data in these diseases. They utilized a combined approach of deep sequencing and panels of tyrosine kinase-specific, small interfering RNAs (siRNA) and small molecule inhibitors to assess the inhibition of cell viability from patient samples. For example, cells from a patient with CNL, who was ultimately found to have a truncating frameshift mutation (S783fs) affecting CSF3R (the G-CSF receptor), exhibited decreased viability with siRNAs against tyrosine kinase nonreceptor 2 (TNK2) and the SRC kinase FGR, and cells from that patient were potently inhibited by dasatinib. Such CSF3R truncation mutations have been previously reported in patients with severe congenital neutropenia, especially at time of leukemic transformation.1-3 Cells from another patient with CNL exhibited sensitivity to the JAK inhibitor ruxolitinib, but no inhibition with dasatinib, with sequencing revealing a membrane proximal CSF3R T618I mutation. Ultimately, of 27 CNL and aCML patients evaluated, 16 (59%) harbored either membrane proximal or truncation CSF3R mutations, with a few patients exhibiting compound mutations of either class. In contrast to their high frequency in CNL and aCML, CSF3R mutations were rare in AML (3/292 cases), and except for one of three cases of early T-cell precursor T-cell ALL, they were not identified in 49 additional cases of B- or T-cell ALL.
Both classes of mutations were able to transform Ba/F3 cells to interleukin-3-independent growth, although the membrane proximal mutations were the more efficient. Ba/F3 cells expressing a truncation mutant expressed high levels of TNK2 and phosphorylated FGR, whereas Ba/F3 cells transformed by CSF3R T618I induced high levels of phosphorylated STAT3 and JAK2, validating the results from the siRNA and drug inhibitor assays. Cells with the S783fs mutation demonstrated higher expression levels of CSF3R, a finding consistent with prior studies showing that truncation mutants result in blockade of receptor internalization. Notably, treatment of a CSF3R T618I-mutated CNL patient with ruxolitinib, 15 mg twice daily, resulted in a marked decrease of neutrophilic leukocytosis and normalization of the platelet count that still persisted a year later.
Figure courtesy of Dr. Jeffrey Tyner; used with permission from the New England Journal of Medicine.
Figure courtesy of Dr. Jeffrey Tyner; used with permission from the New England Journal of Medicine.
In Brief
Use of an integrated functional and genetic sequencing strategy has unmasked highly recurrent, druggable CSF3R mutations in CNL and atypical CML. The role of the G-CSF—CSF3R axis in promoting the growth, differentiation, and survival of myeloid cells is consistent with the finding of neutrophilic leukocytosis in hematologic malignancies with activating CSF3R mutations. Truncation mutations result in increased levels of CSF3R, signal predominantly through SRC kinases, and exhibit drug sensitivity to SRC kinase inhibitors such as dasatinib. In contrast, CSF3R membrane proximal mutations strongly activate the JAK/STAT pathway and are sensitive to JAK kinase inhibitors such as ruxolitinib (Figure). Clinical responses and CSF3R genotype will need to be prospectively validated in a larger cohort of patients. Undoubtedly, CSF3R mutation status will be incorporated into World Health Organization diagnostic criteria for CNL and atypical CML and should help dissect the clinical basis of neutrophilias whose etiology is unclear.