Thrombin provides the momentum for clot formation. As little as 1 percent of the total thrombin generated during the coagulation process is needed to activate all of the components of the system, including platelets, clotting factors (fibrinogen and factors V, VIII, and XI), and the anticoagulant, protein C.1 Increasingly, assays of thrombin generation, the so-called “global” assays, are being recognized as more precise measures of coagulation that offer qualitative and quantitative information about clot formation that is more integrative than the standard, one-dimensional aPTT and PT clotting assays. These global assays, which include thrombin generation and thromboelastometry (ROTEM), yield results that correlate more precisely with clinical bleeding phenotypes than assays that are in clinical use currently. So what exactly do the thrombin generation and the ROTEM assays measure, and how do the findings apply to the management of clinical bleeding disorders?
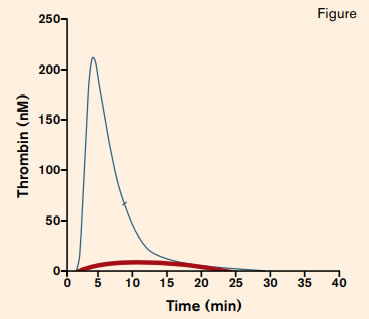
Thrombin Generation in a Patient With Severe Hemophilia A. Adapted with permission from Thrombosis and Haemostatis (Marlu, R et al. Thromb Haemost 2012; 108: 217-224).
Thrombin Generation in a Patient With Severe Hemophilia A. Adapted with permission from Thrombosis and Haemostatis (Marlu, R et al. Thromb Haemost 2012; 108: 217-224).
In the current paper, Young and colleagues provide an overview of the application of global assays to clinical management of congenital hemophilia. While it has long been recognized that the correlation between disease severity based on clotting factor assays and clinical bleeding phenotype is imprecise, management is nonetheless determined largely using data generated by such assays. Further, because of the lack of correlation between factor assay results and bleeding propensity, clinical trial outcomes in patients with hemophilia are based on patient-reported bleeding. Clearly, more objective outcome measures are needed. Thrombin generation measures the lag time to clot formation, the peak thrombin generated, and the endogenous thrombin potential (ETP) as determined by the area under the curve (Figure, blue line). In a patient with severe hemophilia A, all three parameters are abnormal, with delayed lag time and greatly reduced peak thrombin generation and ETP observed (in this case, the thrombin generation assay plot appears as a nearly flat line).2 (Figure, red line) After factor VIII treatment, all three parameters improve. By comparing the parameters of thrombin generation post-infusion with those of a normal control, the dose of factor VIII that most closely simulates normal thrombin generation for a given hemophilia patient can be determined. Using this approach, hemophilia management can be individualized (Table).3
Thrombin generation assays can also be used to monitor hemostatic efficacy in hemophilia patients with acquired inhibitors who require bypass agents such as recombinant factor VII or factor eight inhibitor bypass activity (FEIBA) to manage bleeding complication or to provide hemostasis support perioperatively (Table). By conducting a series of spiking experiments with increasing concentrations of recombinant factor VII or FEIBA, it is possible to determine the minimal dose of the optimal bypass agent required to attain peak thrombin generation for a given inhibitor patient.4,5 This information could be used to determine the dose and dose frequency of bypass therapy required to maintain peak thrombin generation throughout the management period. Lack of clinical availability of such an assay is a particularly pressing issue, as currently there are no approved laboratory methods by which the efficacy of bypass therapy can be measured. Similar spiking experiments with clotting factor concentrates, anti-fibrinolytic agents, and anticoagulants can also be conducted by using the ROTEM assay. Thromboelastometry measures time to clot initiation, clot propagation, and clot firmness. By adding different activators, the ROTEM assay can be adapted to measure clot stability after treatment with various products. For example, tissue plasminogen activator can be added to induce fibrinolysis to measure the effects of treatment of a hemophilia patient with tranexamic acid.
Table.Potential Future Applications of "Global Assays" in Hemophilia Management
| Potential Use of Global Assays . | Role . | Application . |
|---|---|---|
| Disease severity and phenotype | Use global assays to predict bleeding risk for each severity of hemophilia | Determine in whom prophylaxis should be initiated early |
| Personalized prophylaxis | Use global assays to determine peak and trough thrombin generation in product-spiked patient plasma samples | Provide individualized dose regimens based on thrombin generation values |
| Efficacy of bypass therapy | Use global assays to determine optimal dose and to monitor coagulation induced by rV11a or activated prothrombin complex concentrates | Tailor dose and monitor hemostasis in inhibitor patients undergoing surgery |
| Efficacy of long-acting factors | Use global assays to assess thrombin generation induced by long-acting clotting factors | Determine optimal dosing and monitoring parameters for long-acting factors |
| Potential Use of Global Assays . | Role . | Application . |
|---|---|---|
| Disease severity and phenotype | Use global assays to predict bleeding risk for each severity of hemophilia | Determine in whom prophylaxis should be initiated early |
| Personalized prophylaxis | Use global assays to determine peak and trough thrombin generation in product-spiked patient plasma samples | Provide individualized dose regimens based on thrombin generation values |
| Efficacy of bypass therapy | Use global assays to determine optimal dose and to monitor coagulation induced by rV11a or activated prothrombin complex concentrates | Tailor dose and monitor hemostasis in inhibitor patients undergoing surgery |
| Efficacy of long-acting factors | Use global assays to assess thrombin generation induced by long-acting clotting factors | Determine optimal dosing and monitoring parameters for long-acting factors |
In Brief
The thrombin generation and ROTEM assays provide the means both to gain insight into the basis of the heterogeneity of clinical bleeding phenotypes and to improve management of patients with hemophilia. These assays will also be valuable in providing objective data upon which to base measurement of outcomes in clinical trials. Standardization is the primary issue that must be addressed before these global assays can be implemented for routine clinical use. Encouragingly, an international study is underway to resolve this issue.6
References
Competing Interests
Dr. Ragni indicated no relevant conflicts of interest.