Meyer JA, Wang J, Hogan LE, et al. Relapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemiaRelapse-specific mutations in NT5C2 in childhood acute lymphoblastic leukemia. Nat Genet. 2013;45:290-294.
More than half of adults and about 20 percent of children who are diagnosed with acute lymphocytic leukemia (ALL) and achieve remission with combination cytotoxic chemotherapy will eventually relapse. Among the many factors contributing to relapse risk in ALL are individual variations in drug metabolism, non-adherence to and intolerance of therapy, and inherent drug resistance of neoplastic subclones. Two groups have now uncovered evidence of a new mechanism of drug resistance: somatic gain-of-function mutations in the NT5C2 gene encoding cytosolic 5’nucleotidase II, an enzyme responsible for dephosphorylation of 6-hydroxypurine nucleotide monophosphates. Such mutations are present in approximately 19 percent of relapsed pediatric T-cell ALL (T-ALL) cases and in a smaller proportion of relapsed pediatric B-cell ALL (B-ALL) cases.
High levels of NT5C2 expression had previously been associated with both worse outcomes and cytarabine (AraC) resistance in acute myeloid leukemia.1 The newly discovered activating mutations accelerate both metabolism and export active metabolites of nucleoside analogue drugs such as 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG), commonly used agents in various ALL treatment regimens recently found to be of particular therapeutic importance in T-ALL.2
Adolfo Ferrando at Columbia University led the first study in which the investigators performed whole-exome sequencing of five pediatric T-ALL cases at the time of diagnosis, remission, and relapse in a study designed to identify new mechanisms of therapy resistance and disease relapse. Among 60 somatic mutations affecting coding regions, 24 were confined to relapsed T-ALL samples. Affected genes included entities known to be involved in disease pathogenesis such as TP53 and NRAS as well as the new finding of mutations affecting NT52C2. Targeted exon sequencing of NT5C2 in 98 additional cases of relapsed T-ALL and 35 cases of relapsed B-ALL identified NT5C2 mutations in 19 percent of relapsed T-ALL cases and 1 of 35 (3%) of relapsed B-ALL cases. NT5C2 mutations could not be identified in any specimens obtained at the time of initial diagnosis, suggesting that the mutations were acquired during therapy or were present at very low levels before selection by purine nucleoside exposure between the time of diagnosis and relapse.
In the second study, William Carroll and colleagues at New York University performed full transcriptome sequencing of 10 matched diagnosis and relapse pediatric B-ALL samples and identified 20 novel non-synonymous mutations unique to the relapse samples, with two out of 10 patients harboring mutations in the NT5C2 gene. Full exon sequencing of NT5C2 in 61 additional relapse samples identified five more cases with recurrent mutations in this gene, giving an overall occurrence of 10 percent in relapsed pediatric B-ALL specimens. Unlike the group at Columbia, Carroll and colleagues were able to show the presence of rare NT5C2-mutated clones in two out of seven diagnostic samples using ultra-deep sequencing, arguing that the resistant clone was present at low levels from the outset of the disease.
Structure-function analysis supports the hypothesis that these are gain-of-function mutations that result in increased NT5C2 enzymatic activity. In addition, treatment of ALL cell lines expressing wild-type and mutant NT5C2 with nucleoside analogs 6-MP and 6-TG demonstrated increased resistance of cells expressing mutant NT5C2 to nucleoside analogs, but not to doxorubicin, gemcitabine, prednisolone, or guanosine arabinoside (AraG) or its FDA-approved methoxy analogue nelarabine, both of which are metabolized distinctly from 6-MP and 6-TG.
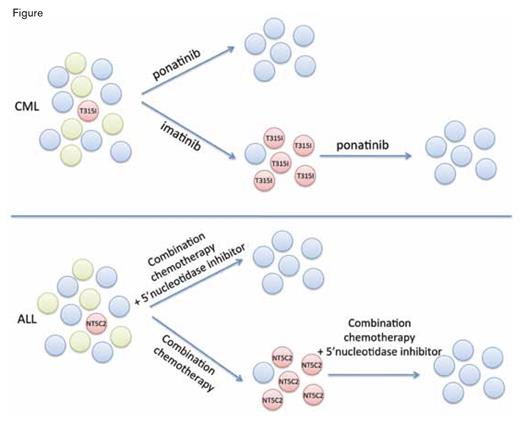
Mechanisms of drug resistance in hematologic malignancies can include selection of resistant clones present at very low levels at the time of diagnosis. For instance, in chronic myeloid leukemia (CML), treatment with a tyrosine kinase inhibitor (TKI) such as imatinib can reduce the dominant neoplastic clone (green circles) and permit recovery of normal hematopoiesis (blue circles), but in some cases TKIs also apply selective pressure allowing outgrowth of preexisting neoplastic subclones harboring ABL kinase mutations such as T315I (red circles), which eventually leads to resistance and treatment failure. Targeting of the resistance clone with a third-generation TKI such as ponatinib can lead to long-term remission; it remains to be seen whether earlier treatment with ponatinib will prevent emergence of resistant clones. Similarly, in ALL, the presence of an activating NT5C2 mutation can lead to relapse during 6-MP maintenance therapy and will require use of adjuvant or non-cross-resistant therapies to overcome.
Mechanisms of drug resistance in hematologic malignancies can include selection of resistant clones present at very low levels at the time of diagnosis. For instance, in chronic myeloid leukemia (CML), treatment with a tyrosine kinase inhibitor (TKI) such as imatinib can reduce the dominant neoplastic clone (green circles) and permit recovery of normal hematopoiesis (blue circles), but in some cases TKIs also apply selective pressure allowing outgrowth of preexisting neoplastic subclones harboring ABL kinase mutations such as T315I (red circles), which eventually leads to resistance and treatment failure. Targeting of the resistance clone with a third-generation TKI such as ponatinib can lead to long-term remission; it remains to be seen whether earlier treatment with ponatinib will prevent emergence of resistant clones. Similarly, in ALL, the presence of an activating NT5C2 mutation can lead to relapse during 6-MP maintenance therapy and will require use of adjuvant or non-cross-resistant therapies to overcome.
In Brief
Together, these studies provide information about a new mechanism underlying relapse in a subset of patients with ALL, paralleling recognized drug resistance mechanisms in other neoplasms (Figure). Incorporation of 5’-nucleotidase inhibitors targeting NT5C2 to initial therapy regimens or to salvage regimens at the time of relapse, or greater use of anti-tumor agents that are not affected by changes in this metabolic pathway, may prevent drug resistance and disease relapse in this vulnerable subset of ALL patients.
References
Competing Interests
Drs. Tothova and Steensma indicated no relevant conflicts of interest.