Students of hematology are taught that hemostasis involves a two-step process. After vascular injury, primary hemostasis consists of platelet adhesion to subendothelial elements followed by platelet aggregation to form a platelet plug. Secondary hemostasis consists of consolidation of the platelet plug by a fibrin clot. Recently, increasing attention has been paid to inflammatory hemorrhage that occurs in the absence of a vascular injury, i.e., without a laceration or rent in the vascular wall.1 Platelets play conflicting roles in inflammatory hemorrhage. They increase inflammation by promoting tissue infiltration by white blood cells. However, they also reduce hemorrhage by nurturing vascular integrity during inflammation.2 Now Boulaftali et al. in the laboratory of Wolfgang Bergmeier at the University of North Carolina at Chapel Hill report that different platelet signaling pathways are involved in controlling hemorrhage due to vascular injury compared with hemorrhage due to inflammation.
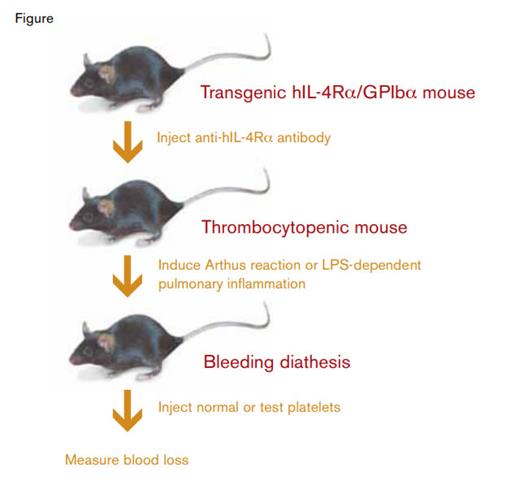
Murine Model System to Evaluate Platelet-Dependent Protection Against Inflammatory Hemorrhage.
Murine Model System to Evaluate Platelet-Dependent Protection Against Inflammatory Hemorrhage.
One possible way to study platelet function would be to transfuse platelets that have been modified in some way into thrombocytopenic mice. For example, the function of a particular signaling pathway could be evaluated by comparing pathway-deficient platelets and normal (wild-type) platelets in their capacity to prevent bleeding in thrombocytopenic mice. But how does one go about producing a thrombocytopenic recipient mouse? Antibodies to platelet glycoproteins, e.g. anti-GPIbα, have been shown to produce severe thrombocytopenia and a bleeding diathesis. However, these antibodies would persist in recipient plasma and result in clearance of the transfused donor platelets. To circumvent this problem, Boulaftali et al. used a clever murine model system. They employed a transgenic mouse created in the laboratory of Jerry Ware3 in which the murine GPIbα platelet surface glycoprotein had been replaced with a fusion protein consisting of the human interleukin-4 receptor-α subunit fused to the transmembrane and cytoplasmic tail of human GPIbα (hIL-4Rα/GPIbα). They found that antibodies to human IL-4Rα produced severe thrombocytopenia in the transgenic hIL-4Rα/GPIbα mice. However, transfusion of wild-type donor platelets, which lack the human IL-4Rα epitope, corrected the thrombocytopenia. Using a laser-induced vascular injury model, they demonstrated that primary hemostasis was normal in thrombocytopenic transgenic hIL-4Rα/GPIbα mice transfused with wild-type platelets.
The authors employed two inflammatory hemorrhage models in the thrombocytopenic transgenic hIL-4Rα/GPIbα mice. In an Arthus reaction model, in which inflammation is immune-complex mediated, mice received intravenous bovine serum albumin (BSA) and intradermal injection of anti-BSA antibodies. The resulting BSA/anti-BSA immune complexes produce a complement-dependent inflammatory response and quantifiable dermal hemorrhage. In a pulmonary inflammation model, mice received lipopolysaccharide (LPS) intranasally. Bleeding in this model was quantitated by measuring hemoglobin in lavage fluid.
In both models, transfusion of wild-type platelets prevented bleeding, setting the stage for analysis of platelets with defective signaling pathways. The role of G-protein-coupled receptor (GPCR) signaling was studied using four types of platelets: 1) platelets deficient in the thrombin receptor Par4; 2) platelets treated with clopidogrel to inhibit the ADP receptor, P2Y12; 3) platelets treated with aspirin to inhibit thromboxane A2 synthesis; and 4) Par4-deficient platelets treated with both clopidogrel and aspirin. All four platelet preparations were defective in producing primary hemostasis following laser-induced vascular injury. However, surprisingly, they were functionally equivalent to wild-type platelets in preventing bleeding in both the Arthus reaction and LPS inflammation models. These results indicate that GPCR signaling is required for normal hemostasis following vascular injury but not following vascular inflammation.
The authors next determined whether signaling via platelet immunoreceptor tyrosine activation motif (ITAM) receptors is required to protect against inflammatory hemorrhage in the thrombocytopenic transgenic hIL-4Rα/GPIbα mice. Mouse platelets express two ITAM receptors: GPVI, which binds collagen and laminin in the extracellular matrix, and C-type lectin–2 (CLEC2), a receptor for podoplanin on the surface of extravascular cells. Four preparations were tested: a) platelets treated with an anti-GPVI monoclonal antibody, JAQ1; b) platelets isolated from Clec2–/– mice; c) JAQ1-treated Clec2–/– platelets; and d) Slp–/–-deficient platelets, which have a defect downstream of ITAM receptors.
In both the Arthus reaction and LPS models, transfusion of JAQ1-treated platelets or Clec2–/– platelets only partially corrected the bleeding diathesis. Furthermore, complete inhibition of ITAM signaling pathways using JAQ1-treated Clec2–/– platelets or Slp76–/– platelets completely failed to correct the bleeding diathesis. These results indicate that maintenance of vascular integrity during inflammation depends on platelet ITAM signaling.
In Brief
The paper by Boulaftali et al. describes a novel model system for studying how platelets protect against bleeding in response both to vascular injury and to inflammation. GPCR-dependent signaling is required for the former, but not the latter. In contrast, ITAM-dependent signaling is required for the latter. The authors speculate that bleeding at sites of inflammation may be an adverse effect of drugs currently under development that target platelet ITAM signaling pathways.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.