Blood performs a remarkable function in response to vascular injury. It rapidly detects the injury and forms around it a new structure, the thrombus, capable of preventing blood loss. The thrombus achieves hemostasis in this pressurized system despite significant shear stress and, except in cases of pathologic thrombus formation, without interrupting blood flow. We have learned much about thrombus formation by dissecting, at a molecular level, the pathways leading to platelet activation and fibrin generation. Considerably less is known about the structural organization and physical characteristics of the thrombus, but available data suggest that a thrombus is not a hodgepodge of randomly distributed platelets and fibrin, but rather a defined structure with a distinct architecture. Yet, how this structure is assembled, in a kinetically and spatially regulated manner, is largely enigmatic.
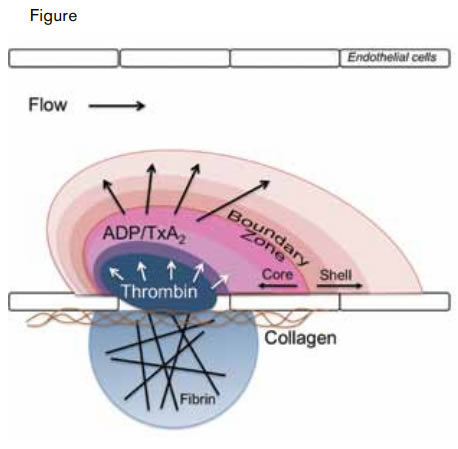
Modeling the Architecture of a Clot. A thrombus is composed of distinct regions including a fibrin cloud, an inner core, and an outer shell. The fibrin cloud is juxtaposed to the endothelium and penetrates the extravascular space in the setting of endothelial compromise. The inner core is the site of active thrombin generation and consists of activated, tightly packed platelets. The outer shell is composed of loosely packed platelets recruited to the thrombus by soluble small molecules such as ADP and thromboxane A2 (TxA2).Stalker TJ et al. Blood. 2013;121:1875-1885.
Modeling the Architecture of a Clot. A thrombus is composed of distinct regions including a fibrin cloud, an inner core, and an outer shell. The fibrin cloud is juxtaposed to the endothelium and penetrates the extravascular space in the setting of endothelial compromise. The inner core is the site of active thrombin generation and consists of activated, tightly packed platelets. The outer shell is composed of loosely packed platelets recruited to the thrombus by soluble small molecules such as ADP and thromboxane A2 (TxA2).Stalker TJ et al. Blood. 2013;121:1875-1885.
Stalker and colleagues at the University of Pennsylvania have now used intravital microscopy to follow the construction of the thrombus over time, to detail certain physical parameters, and to define the platelet signaling pathways that direct its organization. First, the authors confirmed previous studies demonstrating that platelet activation (as detected by platelet P-selectin expression) during thrombus formation is localized to a central core, surrounded by a shell of unactivated platelets.1 They then evaluated the packing density of the thrombus by studying the penetration of fluorescently labeled albumin and dextrans. These studies demonstrated that large molecules penetrated the core poorly but that they flowed relatively easily through the loosely packed outer shell. Formation of the tightly packed core required the generation of thrombin and was blocked by the thrombin inhibitor hirudin. Platelet-platelet interactions were also essential for formation of the tightly packed core. Core formation was impaired in mice lacking semaphorin 4D, a molecule that mediates tight platelet-platelet adhesion. In contrast, augmentation of Gαi2 signaling, which couples to the ADP receptor P2Y12, resulted in an increase in the shell of the thrombus without substantially affecting the core size. And in corollary experiments, inhibition of P2Y12 signaling blocked shell formation without affecting the core. Fibrin formation was observed to infiltrate the extravascular space in those injuries that penetrated the vascular endothelium (Figure). Collectively, these studies describe a clot structure in which fibrin is juxtaposed to endothelial or underlying matrix and interspersed in a core of tightly packed platelets that is surrounded by a shell of more loosely packed platelets (Figure).
In Brief
Understanding the architecture of a thrombus could have clinical implications in the treatment of thrombotic disease. It is possible, for example, that targeting pathways responsible for formation of the thrombus shell will impair occlusive thrombosis without substantially impairing hemostasis. The efficacy and safety of shell-targeted therapies that inhibit thromboxane-mediated signaling (e.g., aspirin) or ADP-mediated signaling (e.g., clopidogrel or ticagrelor) may support this notion. On the other hand, ADP-mediated signaling is known to function in hemostasis,2 and a relationship between thrombus architecture and clinical outcomes remains to be explored. Understanding the architecture of clots in different pathologic scenarios and in different vascular beds may show differences in thrombus structure that reveal which regions of the clot are best to target and which are best to preserve. Ultimately, in providing a new set of parameters by which to evaluate the effects of anticoagulants on clot formation, the studies by Stalker et al. could facilitate our efforts to coerce blood to build safer clots.
References
Competing Interests
Dr. Flaumenhaft indicated no relevant conflicts of interest.