1. Instructor, Hematologic Malignancies and Cellular Therapy, Duke University Medical Center
2. Professor of Medicine and Immunology, VA & Duke University Medical Centers
Dr. Friedman received research funding from Rhizen Pharmaceuticals and TG Therapeutics, both of which have been developing a PI3Kδ inhibitor.
Dr. Weinberg indicated no relevant conflicts of interest.
Over the past several years, excitement has been building surrounding the clinical and therapeutic importance of B-cell receptor (BCR) signaling in chronic lymphocytic leukemia (CLL) and other B-cell lymphoproliferative disorders. Small molecule inhibitors of kinases that participate in BCR signaling pathways, specifically inhibitors of Bruton tyrosine kinase (BTK) and the delta isoform of phosphoinositol 3-kinase (PI3Kδ), have received the most attention. The results of recent clinical studies using these agents have demonstrated efficacy and low toxicity in CLL patients with relapsed/refractory disease and in those with high-risk molecular abnormalities. Given the impressive results thus far, will these agents transform the care of CLL patients much as imatinib and other tyrosine kinase inhibitors have done for patients with chronic myeloid leukemia (CML)?
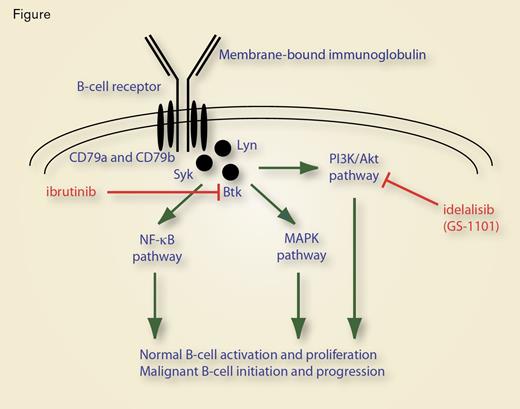
The BCR Consists of Two Parts: the Ligand-Binding Moiety and the Signal-Transduction Moiety.
The BCR Consists of Two Parts: the Ligand-Binding Moiety and the Signal-Transduction Moiety.
BCR signaling is biologically important for normal B-cell activation and proliferation, as well as for initiation and progression of B-cell lymphoid malignancies. The BCR consists of two parts: the ligand-binding moiety and the signal-transduction moiety (Figure). The ligand-binding moiety (the portion of the receptor that recognizes antigen) is, in essence, a membrane-bound antibody that is integrated into the lipid bilayer of the plasma membrane through a hydrophobic transmembrane domain within the immunoglobulin heavy chain. The signal-transduction moiety is a disulfide-linked heterodimer (CD79). The cytoplasmic tail of each of the two chains (CD79a and CD79b, also called Igα and Igβ, respectively) contains an immunoreceptor tyrosine-based activation motif (ITAM). Binding of antigen to the membrane-associated immunoglobulin triggers phosphorylation of ITAM tyrosine residues by the kinases Lyn and Syk that initiates a second messenger cascade through activation of Syk, Lyn, and Btk, with subsequent propagation through PI3K/Akt, MAPK, and NF-κB pathways, resulting in B-cell activation and proliferation (Figure).1
In B-cell lymphoid cancers, BCR-activated downstream signaling pathways are involved in both disease initiation and disease progression. The central role that Helicobacter pylori plays in gastric MALT lymphomagenesis is an example of the importance of signaling through the B-cell receptor in disease initiation. In this case, lymphoma pathogenesis appears to be a consequence of chronic antigenic stimulation of gastric B cells by H. pylori in a process facilitated by reactive T-cells. Antigenic stimulation via the BCR causes oligomerization of BCL10 and MALT1, which ultimately activates the NF-κB pathway and stimulates gastric B-cell proliferation (Figure). In time, clonal expansion arises as a consequence of genetic alterations (particularly translocations that amplify the expression of BCL10 or MALT1), leading to an antigen-independent, malignant state. Thus, antigen-independent BCR signaling drives the oncogenic process.2
In CLL, immunoglobulin heavy chain usage is restricted,3 implying that exposure to particular antigens (either foreign or self) initiates leukemogenesis. However, as in the example of gastric MALT lymphoma, actual leukemic transformation is believed to be an antigen-independent process in which downstream pathways that propagate BCR signaling are constitutively active or amplified, and the involved kinases are overexpressed when compared with normal B cells.1 This aberrant signaling cascade underlies the malignant cells’ proliferative/survival advantage, particularly when combined with additional genetic alterations that affect expression of p53, ATM, miR-15a, miR16-1, Notch1, and SF3B1.4
Our increasingly nuanced understanding of CLL biology, particularly as it pertains to BCR signaling, suggests that there is therapeutic potential in inhibiting the BCR itself or inhibiting the downstream kinase signaling cascade. Because these kinases are overactive or overexpressed in CLL compared with normal lymphocytes, such inhibitor therapy should have a favorable therapeutic index.
Currently, the most active therapeutic regimens used for treatment of CLL are combinations of conventional chemotherapy together with the monoclonal anti-CD20 antibody rituximab. In both initial and relapsed treatment settings, these regimens are highly effective, with excellent overall response rates.5,6 However, efficacy is compromised in the subset of patients with loss of normal p53 activity due to deletion of chromosome 17p or somatic mutation of p53.6,7 In addition, these regimens are associated with significant morbidity as a result of myelo- and immune-suppression. Such toxicity often necessitates dose reduction or truncation of treatment and discourages use in elderly patients. Thus, new treatments that may hold benefit for patients with high-risk disease and that have a favorable therapeutic index are needed.
Ibrutinib (PCI-32765) is an oral BTK inhibitor (Figure). Results of a phase Ib/II study suggest that the drug is active in patients with relapsed/refractory disease.8,9 Notably, a temporary increase in lymphocytosis, beginning after about one week of treatment and typically resolving after several treatment cycles, is observed in patients treated with ibrutinib (and other inhibitors of BCR signaling). The increase in lymphocytosis is thought to be due to redistribution of CLL lymphocytes from the lymph nodes into the peripheral circulation.8,9 The temporary rise in circulating CLL lymphocytes introduces a problem in assessment of response to treatment, because criteria for complete and partial responses require resolution or reduction in both lymphadenopathy and lymphocytosis.10 To resolve this issue, investigators testing BCR signaling inhibitors report results based on the strict definitions of response, but also report the fraction of patients who have a nodal response of ≥ 50 percent with co-existing lymphocytosis.
Updated results of the phase Ib/II clinical trial of ibrutinib monotherapy were presented at the 2012 ASH Annual Meeting (abstract 189). Treatment-naïve patients ≥ 65 years old, patients with relapsed/refractory disease, and patients with high-risk disease were included in the study that enrolled 116 patients who were treated with either 420 or 840 mg of ibrutinib daily until disease progression. Seven patients discontinued therapy due to adverse events. The majority of adverse events were ≤ grade 2. With a median follow-up period of 16 months, the overall response rate ranged between 50 and 71 percent for the three different cohorts, with between 10 and 29 percent achieving a partial remission with lymphocytosis.
Idelalisib (GS-1101 or CAL-101) is an oral inhibitor of PI3Kδ, another mediator of BCR signaling (Figure). In a phase I study of single-agent idelalisib, originally presented at the 2010 ASH Annual Meeting (abstract 55), 37 relapsed or refractory CLL patients were treated either daily or twice daily with escalating doses of idelalisib for up to 12 months. This agent was well tolerated. Partial responses were seen in 11 patients, and an additional 18 patients had ≥ 50 percent reduction in lymph node size accompanied by an increase of lymphocytosis of > 50 percent. Thus, 29/37 (78%) of these heavily pretreated patients responded to treatment with idelalisib.
Researchers have also begun to test both ibrutinib and idelalisib in combination with conventional chemotherapy and/or anti-CD20 immunotherapy in patients with CLL. This combination strategy would be expected to improve the overall response rate compared with either compound alone and to reduce the extent and duration of lymphocytosis caused by ibrutinib or idelalisib. A phase II study of ibrutinib and rituximab in patients with either high-risk cytogenetics or somatic mutations or with relapse within 36 months after initial therapy found that 17 of 20 patients achieved a partial remission at three months and that the remaining three patients had nodal responses with lymphocytosis.11 A phase II study of the combination of idelalisib with rituximab and/or bendamustine demonstrated complete responses in 7 percent of patients treated with the combination of idelalisib, bendamustine, and rituximab, while no complete responses were observed in the other two treatment groups. However, between 78 and 82 percent of patients in the different treatment groups achieved a partial response.12
Other inhibitors of BCR signaling are undergoing evaluation in pre-clinical and clinical studies. At the 2012 ASH Annual Meeting, the results of in vivo or in vitro studies of at least five different PI3K/Akt inhibitors were presented (abstracts 3928, 3663, 3914, 2907, and 1803) and results of a phase I study of the BTK inhibitor AVL-292 in the treatment of CLL was presented at the 2012 American Society of Clinical Oncology Annual Meeting (abstract 8032). In addition, ibrutinib and idelalisib appear to have clinical activity in other B-cell malignancies, such as mantle cell lymphoma, subtypes of diffuse large B-cell lymphoma, and indolent lymphoma.
The results of clinical studies involving ibrutinib and idelalisib, along with the development of other kinase inhibitors of BCR signaling pathways, support the concept that a detailed understanding of the pathobiology of CLL can lead to development of safe, effective targeted therapy. Because these agents are well tolerated, patients may be able to continue therapy indefinitely. But will extended treatment translate into deeper, more durable clinical responses or even into cytogenetic or molecular remissions as are observed in patients with CML treated with tyrosine kinase inhibitors? Given the range of genetic complexity in CLL, such a clinical response could be expected in some patients, but likely not in all. Nonetheless, the BCR signaling inhibitors show promise in the treatment of lymphoproliferative neoplasms, particularly CLL, justifying additional basic and clinical studies to understand the full potential of this therapeutic modality.