“Between animal and human medicine there is no dividing line – nor should there be. The object is different but the experience obtained constitutes the basis of all medicine.” – Rudolf Virchow (1821-1902)1
In support of Virchow’s perspective, a number of notable advances in hematology have their origins in observations made in animals. For example, cats infected with feline leukemia virus C (FLVC) develop red cell aplasia, and investigation of the pathobiology of this process led to identification of the receptor for FLVC (FLVCR1) as a cytoplasmic heme exporter.2-4 Free heme is toxic, and FLVCR protects erythroid progenitors from injury by exporting, from the cytoplasm, heme that is produced in excess of that required for pairing with globin, cytochromes, and other proteins that use heme as a prosthetic group (Figure). But the heme biosynthetic pathway ends in the mitochondria (Figure), so how does newly synthesized heme move from the mitochondria to the cytoplasm? The answer to that question can again be traced to the study of cats, as Deborah Chiabrando and colleagues, in the laboratory of Emanuela Tolosano at the University of Torino, have identified an isoform of FLVCR1 that mediates efflux of mitochondrial heme into the cytoplasm.
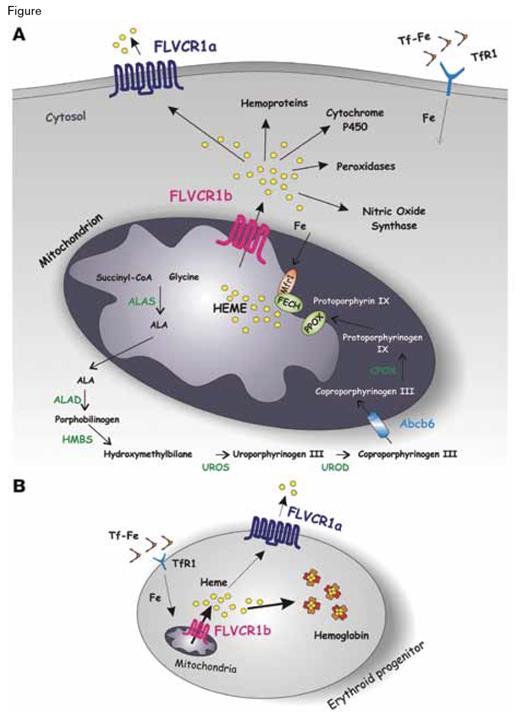
A Model for FLV CR1 Isoforms Function. A) A schematic representation of heme biosynthesis is shown. Transferrin-bound iron (Tf-Fe) is taken up by cells through transferrin receptor 1 (TfR1), and iron is transferred to the mitochondrion for heme biosynthesis. It was reported that the mitochondrial iron importer MITOFERRIN1 (MFRN1) and ferrochelatase (FECH, the enzyme that catalyzes incorporation of iron into protoporphyrin IX to form heme) are part of the same complex in the inner mitochondrial membrane. FLVCR1b could work in association with this complex to allow heme export out of the mitochondrion for incorporation into new hemoproteins. Heme not used for hemoprotein synthesis is exported out of the cell through the cell-surface isoform FLVCR1a.B) During erythroid differentiation, the expression of FLVCR1b in the mitochondrion regulates heme export into the cytosol, allowing hemoglobinization of erythroid precursors. At the cell membrane, FLVCR1a regulates the export of heme in excess. The data reported by Chiabrando et al. suggest that decreased expression of the membrane heme exporter FLVCR1a and increased expression of FLVCR1b are fundamental for proper differentiation of erythroid progenitors.The Mitochondrial Heme Exporter FLVCR1b Mediates Erythroid Differentiation. Chiabrando D, Marro S, Mercurio S, et al. J Clin Invest. 2012; 122(12):4569–4579, doi:10.1172/JCI62422.
A Model for FLV CR1 Isoforms Function. A) A schematic representation of heme biosynthesis is shown. Transferrin-bound iron (Tf-Fe) is taken up by cells through transferrin receptor 1 (TfR1), and iron is transferred to the mitochondrion for heme biosynthesis. It was reported that the mitochondrial iron importer MITOFERRIN1 (MFRN1) and ferrochelatase (FECH, the enzyme that catalyzes incorporation of iron into protoporphyrin IX to form heme) are part of the same complex in the inner mitochondrial membrane. FLVCR1b could work in association with this complex to allow heme export out of the mitochondrion for incorporation into new hemoproteins. Heme not used for hemoprotein synthesis is exported out of the cell through the cell-surface isoform FLVCR1a.B) During erythroid differentiation, the expression of FLVCR1b in the mitochondrion regulates heme export into the cytosol, allowing hemoglobinization of erythroid precursors. At the cell membrane, FLVCR1a regulates the export of heme in excess. The data reported by Chiabrando et al. suggest that decreased expression of the membrane heme exporter FLVCR1a and increased expression of FLVCR1b are fundamental for proper differentiation of erythroid progenitors.The Mitochondrial Heme Exporter FLVCR1b Mediates Erythroid Differentiation. Chiabrando D, Marro S, Mercurio S, et al. J Clin Invest. 2012; 122(12):4569–4579, doi:10.1172/JCI62422.
Examination of the DNA structure of FLVCR1 suggested to the authors the possibility of alternatively spliced transcripts, and their hypothesis was confirmed when they ultimately identified an isoform (that they named FLVCR1b) that consists of amino acids 277-555 of FLVCR1 (renamed FLVCR1a in their paper to distinguish it from the newly discovered FLVCR1b). In mice, Flvcr1b is ubiquitously expressed, with the highest transcript levels in the brain, heart, muscle, spleen, and bone marrow. When overexpressed in vitro, Flvcr1b shows a strikingly different sub-cellular localization compared with Flvcr1a. Flvcr1b has a mitochondrial targeting sequence and is found enriched in mitochondria, whereas Flvcr1a is a plasma membrane protein (Figure). Overexpression of Flvcr1b leads to intracellular heme accumulation, while silencing of expression results both in heme accumulation exclusively in the mitochondria and in termination of erythroid differentiation. Thus, normal erythropoiesis depends upon FLVCR1b-mediated regulation of mitochondrial heme efflux without which hemoglobin and other hemoproteins cannot form (Figure). Remarkably, mice lacking Flvcr1a but expressing Flvcr1b have normal erythropoiesis. The Flvcr1a-/Flvcr1b+ mice are characterized phenotypically by edema, hemorrhage, and skeletal abnormalities. These observations suggest that the block in erythroid differentiation observed in the original Flvcr1-/- knockout model (that eliminated both isoforms)4 was due to aberrant regulation of mitochondria heme regulation due to absence of Flvcr1b function rather than to cytoplasmic accumulation of heme due to loss of Flvcr1a activity. On the other hand, the edema and hemorrhage that characterizes the Flvcr1a-/Flvcr1b+ model may be a consequence of endothelial cell injury due to cytosolic accumulation of heme. This same process may account for the observed skeletal deformities as endothelial cell injury could lead to tissue hypoxia, thereby impairing cartilage development.
In Brief
The exquisite control of heme metabolism and the potential toxicities associated with excess heme accumulation are highlighted by this paper (Figure). The hypochromic, microcytic anemias that are familiar to all hematologists are due to abnormal of production of heme (e.g., iron deficiency) or globin (e.g., thalassemia). But are there anemias in humans that are due to dysregulation of heme trafficking? If so, what is the clinical phenotype? Is there a human counterpart of the red cell aplasia that characterizes infection of cats with feline leukemia virus? To answer these questions, an understanding of the fundamental mechanisms involved in heme homeostasis is essential. The rigorous, imaginative studies of Chiabrando and colleagues have further defined the participants in this intricate process.
References
Competing Interests
Dr. Vercellotti indicated no relevant conflicts of interest.