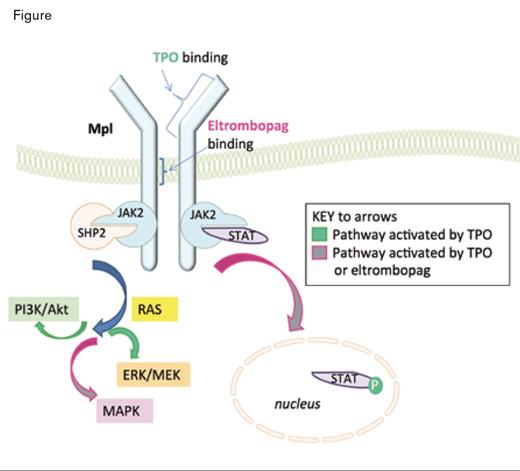
Thrombopoietin (TPO) is the growth factor that stimulates platelet production through interaction with its receptor, Mpl, on megakaryocytes. Surprisingly, patients with immune thrombocytopenia (ITP) exhibit a relative deficiency of TPO and respond to exogenous stimulation by TPO receptor agonists with increased platelet counts. Two TPO mimetics, romiplostim and eltrombopag, have been FDA-approved for the treatment of ITP in patients with an insufficient response to corticosteroids, intravenous immune globulin, or splenectomy; both bind to c-Mpl at a site distinct from the TPO binding site, and neither shares homology with TPO. Eltrombopag is an oral drug that activates the Jak-Stat and MAPK pathways (Figure). A phase III trial of eltrombopag versus placebo demonstrated its capacity to improve platelet counts, reduce bleeding occurrences, and improve quality of life in patients with chronic ITP.1 Eltrombopag was also recently granted FDA approval in November 2012 for patients with hepatitis C, as it effectively supports platelet counts during treatment with interferon.
In contrast to patients with ITP, patients with aplastic anemia exhibit markedly elevated TPO levels, but they still have thrombocytopenia. There are few options for patients with aplastic anemia who have relapsed after immunosuppressive therapy (IST) with anti-thymocyte globulin, cyclosporine, and steroids. The salvage therapies include a second course of IST or an allogeneic stem cell transplant. The former has a poor response rate, and the latter, a high mortality rate, especially for those who do not have a matched family donor.
As has been extensively studied, thrombopoietin receptors are expressed by primitive hematopoietic stem cells, and TPO is a critical cytokine for ex vivo stem cell expansion and lentiviral gene therapy.2 In addition, eltrombopag successfully expands cord blood stem cells in vitro.3 Together, these observations suggested that TPO agonists could be therapeutically active in diseases such as aplastic anemia in which the hematopoietic stem cell pool is depleted.
Thrombopoietin (TPO) and Eltrombopag Bind to Distinct Sites on Mpl. TPO binding leads to activation of the JAK/STAT, PI3K/Akt, MEK, and MAPK pathways, and eltrombopag binding leads to activation of the JAK/STAT and MAPK pathways.
Thrombopoietin (TPO) and Eltrombopag Bind to Distinct Sites on Mpl. TPO binding leads to activation of the JAK/STAT, PI3K/Akt, MEK, and MAPK pathways, and eltrombopag binding leads to activation of the JAK/STAT and MAPK pathways.
Dr. Cynthia Dunbar’s group at the National Institutes of Health conducted a non-randomized, phase II study of eltrombopag in patients with severe aplastic anemia and severe persistent thrombocytopenia who had failed to respond to immunosuppressive therapy. They successively enrolled 25 adult patients and initiated eltrombopag at a dose of 50 mg daily, the typical starting dose for ITP. The dose escalated every two weeks by 25 mg if the platelet count remained below 20,000/mm3 , until a maximum daily dose of 150 mg was reached. All but one patient reached this maximum dose. Response was assessed at 12 weeks as follows: 1) platelet response defined as an increase in platelet count by 20,000 or freedom from platelet transfusions for eight weeks if previously dependent; 2) red cell response defined as an increase in hemoglobin by 1.5 g/dl or reduction by four units of red cells transfused in eight weeks as compared with the eight weeks prior to enrollment; and 3) neutrophil response defined as a rise in count to ≥ 500/mm3 or if < 500 then at least doubling the count. Forty-four percent of the patients responded with improved production in at least one cell lineage. All 25 patients were dependent on platelet transfusions prior to treatment, and nine were able to discontinue them. The average increase of the platelet count was 44,000/μl. Nine patients had a neutrophil response, and six had an erythroid response. Higher reticulocyte count was one characteristic that predicted a favorable response. Of the four patients who had a response of at least eight months, three attained normal bone marrow cellularity. None of the patients’ bone marrows exhibited the reticulin fibrosis seen in some ITP patients treated with eltrombopag. The severe adverse events included abdominal pain due to gastroparesis, skin rash on cephalosporin, febrile neutropenia, and gingival bleeding. Seven of 11 patients with a response remained on treatment for 16 months. One patient who stopped treatment after nine weeks because of development of a cataract exhibited a continued response for 16 months.
Mice deficient in mpl (the murine thrombopoietin receptor) exhibit bone marrow aplasia,4 and humans with congenital amegakaryocytic thrombocytopenia develop multi-lineage marrow failure.5 Moreover, patients with familial aplastic anemia have recently been found to have a defect in Mpl.6
The other thrombopoietin mimetic, romiplostim, has also been investigated in a randomized, double-blind, placebo-controlled trial for low or int-1 risk myelodysplastic syndrome with thrombocytopenia.7 There was sustained improvement in platelet counts among the romiplostim-treated patients, but the study was stopped prematurely due to possible increased incidence of progression to acute myeloid leukemia. The most recent analysis of the data, presented at the 2012 ASH Annual Meeting, however, did not show a statistically significant difference in progression to AML between romiplostim and placebo. Nonetheless, the hypothetical risk of transformation induction remains, as another recent study demonstrated elevated Mpl expression in some leukemia cells with t(8;21) that produces the Runx1-ETO fusion gene, and thrombopoietin-mediated signaling via PI3K-Akt led to development and maintenance of AML in a mouse model expressing Runx1-Eto.8 In fact, two non-responding patients in the eltrombopag trial exhibited clonal evolution with development of monosomy 7, and one of them progressed to AML. Thus, a key objective in utilizing thrombopoietin mimetic therapy for aplastic anemia will be to stimulate normal hematopoiesis without potentiating leukemogenesis.
In Brief
The thrombopoietin receptor’s expression by hematopoietic stem cells provides a new therapeutic target for acquired aplastic anemia. Eltrombopag’s potency in enhancing blood cell production in this setting may enable broader applications of the thrombopoietin mimetics in acquired and familial bone marrow failure. Additional studies are needed to optimize patient selection and establish long-term safety given the theoretical and experimental potential for expansion of dysplastic or leukemic clones.
References
Competing Interests
Dr. Becker indicated no relevant conflicts of interest.