The colony-stimulating factors, granulocyte/macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), and granulocyte colonystimulating factor (G-CSF), were originally defined as hematopoietic cell growth factors. Subsequently, CSFs have been found to have complex, pleiotropic effects in inflammation and in other states.1 In most murine models of inflammation and autoimmunity (e.g., experimental autoimmune encephalomyelitis, nephritis, and arthritis), CSF depletion results in the suppression of the disease state. These findings, which are consistent with a proinflammatory function of CSFs, have led to clinical trials of anti-GM-CSF and anti-M-CSF antibodies in rheumatoid arthritis.
Prior to the present study by Rauch et al. from the laboratory of Filip Swirski, GM-CSF was thought to be produced by non-hematopoietic cells, macrophages, or T cells. However, the authors examined murine GM-CSF expression by flow cytometry and made the surprising observation that in response to lipopolysaccharide (LPS), GM-CSF was found mainly in a splenic B-cell subpopulation. Consistent with this interpretation, immunofluorescence of spleen sections from LPS recipients co-localized GM-CSF expression to red pulp cells that coexpressed the B-cell markers, IgM, B220, and PAX5. Subsequent extensive characterization by flow cytometry revealed that GM-CSF expressing cells were IgMhigh, CD43high, CD93+, CD138+, VLA4high, LFA1high, CD284+, CD23low, IgDlow, and CD21low. In addition to GM-CSF, the cells under investigation secreted IgM and IL-3, but not pro–IL-1β, IL-6, or tumor necrosis factor–α. This set of phenotypic markers defined these cells as a unique mature B-cell population. The authors named the cells “innate response activator B cells” (IRA B cells) because of the known role of GM-CSF in activating innate leukocytes.
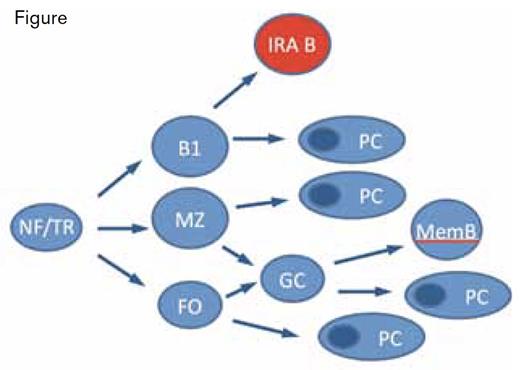
IRA B-Cell Development. NF, newly formed; TR, transitional; FO, follicular; MZ, marginal zone; B1, B1 cell; memB, memory B cell; and PC, plasma cell.Adapted with permission from Martin F, Kearney JF. Immunol Rev. 2000;175:77.
IRA B-Cell Development. NF, newly formed; TR, transitional; FO, follicular; MZ, marginal zone; B1, B1 cell; memB, memory B cell; and PC, plasma cell.Adapted with permission from Martin F, Kearney JF. Immunol Rev. 2000;175:77.
After maturation, B lymphocytes either recirculate through secondary lymphoid organs as part of a long-lived pool (follicular cells) or join more static compartments in the marginal zone of the spleen (MZ B cells) or peritoneal and pleural cavities (B1 B cells).2 The authors performed transcriptional analysis on isolated IRA B cells and using hierarchical clustering and principal component analysis found that IRA B cells fit into a population separate from transitional, follicular, marginal zone, B1, or plasma cells. Adoptive transfer and parabiosis experiments demonstrated that peritoneal B1 cells give rise to IRA B cells (Figure), of which B1a cells were the dominant precursor. B1a cells in culture produced multiple cell types, including IRA B cells. MZ B cells use the adhesive ligands VLA-4 and LFA-1 to remain lodged in the spleen. Similarly, IRA B cells, which express VLA-4 and LFA-1, are dislodged from the spleen upon injection of anti-VLA-4 and anti-LFA-1 antibodies. These and other experiments described by the authors are consistent with a model in which IRA B cells are the progeny of peritoneal B1a B cells and migrate to and take up residence in the spleen.
To assess the functional significance of IRA B cells, the authors employed a murine cecal ligation and puncture (CLP) sepsis model. They produced mixed chimeras by reconstituting lethally irradiated mice with bone marrow from a B-cell-deficient mouse called μMT and from a GM-CSF– deficient (Csf2–/–) mouse. The B cells of these mice (called GM/μMT chimeras) cannot produce GM-CSF. The mortality of GM/μMT mice was significantly greater than that of normal mice in the CLP model. Additionally, the peritoneal cavities of GM/μMT chimeras had more neutrophils, and the mice developed an IL-1β, IL-6, and TNFα cytokine storm. This cytokine profile is associated with a defect in bacterial clearance. Neutrophils from the GM/μMT chimeras also phagocytosed bacteria poorly and had higher levels of bacteremia.
In Brief
The paper by Rauch et al. describes the discovery of a novel B cell, the IRA B cell, which expresses biologically significant levels of GM-CSF in a proinflammatory setting. The results indicate that, while inhibition of GM-CSF may be beneficial in some settings, such as chronic inflammatory states, GM-CSF may be protective in other settings, such as sepsis. The study also raises the question of how IRA B cells may participate in other infectious and inflammatory diseases.
References
Competing Interests
Dr. Lollar indicated no relevant conflicts of interest.