Feys HB , Roodt J, Vandeputte N, et al. Inhibition of von Willebrand factor – platelet glycoprotein Ib interaction prevents and reverses symptoms of acute acquired thrombotic thrombocytopenic purpura in baboonsInhibition of von Willebrand factor – platelet glycoprotein Ib interaction prevents and reverses symptoms of acute acquired thrombotic thrombocytopenic purpura in baboons. Blood. 2012:120:3611-3614.
Plasma exchange has greatly reduced the morbidity and mortality of thrombotic thrombocytopenic purpura (TTP). Yet, even when combined with corticosteroids and/or rituximab (Table), not all patients respond and complications related to the exchange procedure remain.1 Thus, better therapeutic approaches are needed.
Table
| Mechanisms of TTP Therapies . | Provides ADAMTS13 Activity . | Reduces Anti-ADAMTS13 Antibody . | Suppresses Immune Response . | Blocks VWF Binding to GP1b . |
|---|---|---|---|---|
| Plasma Exchange | + | + | - | - |
| Steroids | - | - | + | - |
| Rituximab | - | - | + | - |
| ALX-0681* | - | - | - | + |
| GBR600* | - | - | - | + |
| Mechanisms of TTP Therapies . | Provides ADAMTS13 Activity . | Reduces Anti-ADAMTS13 Antibody . | Suppresses Immune Response . | Blocks VWF Binding to GP1b . |
|---|---|---|---|---|
| Plasma Exchange | + | + | - | - |
| Steroids | - | - | + | - |
| Rituximab | - | - | + | - |
| ALX-0681* | - | - | - | + |
| GBR600* | - | - | - | + |
*Based on studies in animals.
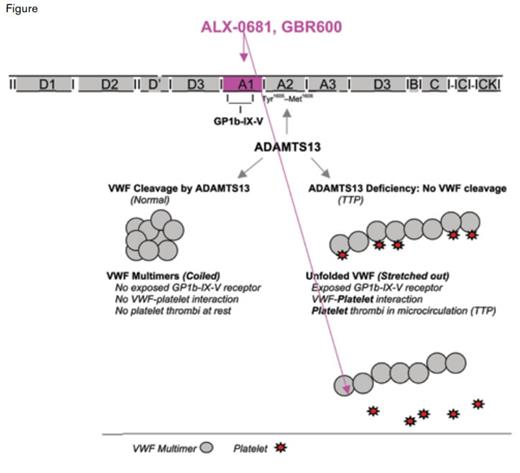
In both the congenital and acquired forms of TTP, deficiency of the metalloprotease ADAMTS13 results in decreased or absent cleavage of high-molecular-weight multimers of von Willebrand factor (VWF).2 These unprocessed ultra-large (UL)-VWF multimers spontaneously bind to platelets forming platelet-rich thrombi that occlude the microvasculature (a pathologic process called thrombotic microangiopathy). It is this process that causes the end-organ damage that accounts for the clinical manifestations of TTP (i.e., thrombocytopenia, microangiopathic hemolytic anemia, renal insufficiency, and mental status changes).
Taking advantage of the known pathobiology of TTP to develop novel approaches to therapy, two teams of investigators independently reported that anti-VWF antibodies prevent signs of the disease in a baboon model of acquired TTP. Experimentally, TTP was induced by injecting animals with 3H9, a monoclonal antibody that blocks ADAMTS13, thus mimicking the acquired form of the human disease. Callewaert and colleagues evaluated the efficacy of nanobody ALX-0681. Nanobodies are modeled after heavy-chain- only Igs found in camels, llamas, alpacas, and other members of the family Camelidae and are much smaller (12-15 kDa) than standard antibodies (150-160 kDa). ALX-0681 is a bivalent humanized nanobody that targets the A1 domain of VWF that binds to platelet GP1b, the VFW receptor on platelets (Figure). Given concomitantly with 3H9, ALX-0681 prevented the onset of TTP; and given subsequent to antibody-mediated induction of TTP, ALX-0681 promoted rapid resolution of thrombocytopenia and hemolytic anemia.
Mechanisms of Thrombotic Thrombocytopenic Purpura Therapies. Deficiency of the metalloprotease ADAMTS13, the cause of TTP, results in loss of proteolytic processing of VWF and plasma accumulation of ultra-large (UL)-VWF multimers. UL-VWF multimers are highly platelet-adhesive and induce thrombi in the micro-vasculature of various organ beds, leading to the clinical manifestations of TTP. When the VWF-platelet interaction is blocked by monoclonal antibodies (ALX-0681 or GBR600) that recognize the site on VWF that binds to the GP1b receptor on platelets, VWF-platelet interaction is blocked, preventing formation of microthrombi and the clinical symptoms of TTP.
Mechanisms of Thrombotic Thrombocytopenic Purpura Therapies. Deficiency of the metalloprotease ADAMTS13, the cause of TTP, results in loss of proteolytic processing of VWF and plasma accumulation of ultra-large (UL)-VWF multimers. UL-VWF multimers are highly platelet-adhesive and induce thrombi in the micro-vasculature of various organ beds, leading to the clinical manifestations of TTP. When the VWF-platelet interaction is blocked by monoclonal antibodies (ALX-0681 or GBR600) that recognize the site on VWF that binds to the GP1b receptor on platelets, VWF-platelet interaction is blocked, preventing formation of microthrombi and the clinical symptoms of TTP.
Feys and colleagues investigated the effects of the humanized murine monoclonal anti-VWF antibody GBR600 and showed it was effective in both preventing and reversing the thrombocytopenia in the baboon model of TTP. The efficacy of GBR600 was shown to correlate with the capacity of the antibody to inhibit VWF activity.
In Brief
The clinical modulation of TTP by anti-VWF monoclonal antibodies demonstrated by Callewaert et al. and Feys et al. provides an exciting new approach to treat TTP, specifically by blocking the interaction between UL-VWF multimers and platelet GP1b in order to prevent formation of pathologic platelet thrombi. Importantly, neither brain CT scans nor post-mortem analysis revealed signs of bleeding in the animals treated with the nanobody ALX-0681.
How applicable are these studies of antibody-induced TTP in baboons to the human disease? First, it is important to note that the baboon model may represent early TTP only, as the targeted inhibition of ADAMTS13 by the monoclonal antibody 3H93 results in thrombocytopenia and hemolytic anemia, but no endorgan disease, including absence of neurologic dysfunction and renal insufficiency in the animal model. Secondly, ADAMTS13 deficiency is alone sufficient to cause thrombocytopenia and microangiopathic hemolytic anemia in the baboon model, with no requirement for exogenous triggers of disease, as is typical in human TTP. Thirdly, while starting anti-VWF therapy early may eliminate signs of the disease, recognition of human TTP may be delayed until neurologic symptoms develop or until clinical symptoms become sufficiently severe for patients to seek medical attention. Thus, it is unclear if the timing of the experimental treatment is informative from a clinical perspective.
If disease can be averted by anti-VWF therapy, better diagnostic tests to recognize disease earlier would be ideal. Alternatively, if the therapy is safe and inexpensive, early treatment might be practical and desirable in patients with suspected, but unproven, TTP. Clearly, additional studies are needed to define the extent to which anti-VWF monoclonal antibodies can affect the natural history of TTP in humans and to determine when in the course of the disease anti-VFW antibody treatment is effective and if it is sufficiently effective as monotherapy or best used in conjunction with standard therapy centered on plasma exchange.
References
Competing Interests
Dr. Ragni indicated no relevant conflicts of interest.