Study Title: A Phase 1b trial of Magrolimab Monotherapy or Magrolimab in Combination With Azacitidine in Participants With Hematological Malignancies
ClinicalTrials.gov Identifier: NCT03248479
Sponsor: Gilead Sciences
Participating Centers: 26 study locations across the United States and one in the United Kingdom
Accrual Goal: 287 participants
Study Design: This is a phase Ib study to evaluate the effects of magrolimab, a first-in-class anti-CD47 antibody in myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML). This study began accrual in 2017 and was designed as a nonrandomized, open-label trial comparing magrolimab both as monotherapy and in combination with azaciditine. The regimen uses a priming dose for magrolimab and subsequent escalation titrated according to levels of anemia to a maximum dose of 30 mg/kg weekly or biweekly, depending on the treatment arm. In combination treatment arms, azaciditine has been administered at the standard 75 mg/m2 on days 1 to 7 of a 28-day cycle. The key eligibility criteria include a diagnosis of relapsed/refractory AML or intermediate to very high risk MDS in which the patient is refractory or intolerant to conventional therapy. A separate arm also includes previously untreated AML that is ineligible for intensive therapy or untreated MDS. Those with transfusion-dependent low-risk MDS are also studied in a separate arm. The study has primarily sought to address aspects of safety and tolerability as well as those of efficacy in these groups, with key measures including complete remission (CR) rates for both AML and MDS as well as duration of CR. For low-risk MDS, red blood cell–transfusion independence is the key metric evaluated. Secondary endpoints include overall response rate (ORR), overall survival (OS), prolonged transfusion independence, progression-free survival, and minimal residual disease levels. Treatment-naïve patients are excluded from the study if there has been any prior exposure to antileukemic therapies (including hypomethylating agents and/or low-dose cytarabine). Prior anti-CD47 therapy or recent stem cell transplantation in the prior six months are also key exclusion criteria.
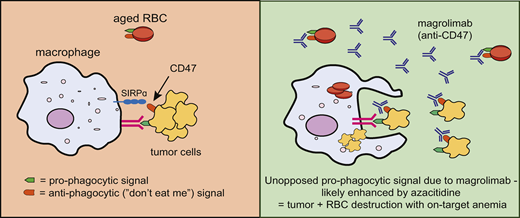
CD47 is highly expressed on tumor cells and aged red cells. This is recognised by SIRPα and represents a “don’t eat me” signal to prevent macrophage phagocytosis. Blocking this CD47 signal with magrolimab enhances macrophage phagocytosis of tumor and red cells, leading to potent anti-tumor activity and also the on-target toxicity of anemia.
CD47 is highly expressed on tumor cells and aged red cells. This is recognised by SIRPα and represents a “don’t eat me” signal to prevent macrophage phagocytosis. Blocking this CD47 signal with magrolimab enhances macrophage phagocytosis of tumor and red cells, leading to potent anti-tumor activity and also the on-target toxicity of anemia.
Results to Date: The results to date seem promising, particularly in high-risk populations such as patients with TP53-mutant AML. The MDS patient data were reported at the European Hematology Association conference in 2020, with 91 percent of evaluable patients with MDS showing evidence of an objective response and 42 percent of patients achieving CR. The CR figure rose to 56 percent after six months of therapy indicating the response is both durable and typically deepens over time. Combination therapy was otherwise generally well tolerated. An update was also presented at the 2020 ASH Annual Meeting for the AML cohort on the trial with evidence of an ORR of 63 percent and CR rate of 42 percent. Importantly, with a purposeful bias for selecting patients with p53-mutant disease, ORR and CR rates were comparable at 69 percent and 45 percent, respectively, with a median OS of 12.9 months — a significant improvement compared to the azacitidine/venetoclax combination for TP53-mutant AML (∼5-7 mo. OS).
Comment: This study represents the first in-human trial of macrophage immune checkpoint therapy to be used in the context of myeloid malignancies, specifically in MDS and AML, correlating with the analogous use of T-cell immune checkpoint therapies in lymphoid and solid organ cancers. To date, immunotherapy approaches in AML have been somewhat disappointing, with only CD33 targeting with gemtuzumab ozogamycin in AML receiving U.S. Food and Drug Administration (FDA) approval. The significance of the magrolimab results to date is reflected in the FDA’s decision to grant Breakthrough Therapy designation in the U.S. as of September 2020. This supports the basis that magrolimab may address a significant area of unmet need for those with a diagnosis of high-risk MDS. Magrolimab has also achieved a Fast Track designation for the treatment of MDS, AML, and a number of lymphoid malignancies. Additionally, the strikingly positive results for those with TP53-mutant AML — a subtype of AML with a particularly poor prognosis — cannot be ignored. Beyond the current study, it will be important to determine whether magrolimab may play a role in TP53-mutant MDS and its potential role in therapy-related AML/MDS. Based on the current results to date in MDS, a double-blinded placebo-controlled randomized phase III trial — the ENHANCE study — is currently underway for those with higher-risk MDS to further corroborate initial findings. This will measure CR rates and duration of CR for the combination of magrolimab with azacitidine versus azacitidine alone. An additional point for future study and clarification is whether magrolimab plus azacitidine might provide superior outcomes in a head-to-head study against venetoclax with azacitidine (though the current results do not necessarily represent superiority), or whether triple therapy could be tested to improve survival in AML. The latter hypothesis is currently being addressed through a phase Ib/II open-label study out of the MD Anderson Cancer Center (NCT04435691). Overall, the results to date are particularly significant because they represent a novel approach in treating myeloid malignancy through harnessing of the immune system, with highly promising preliminary data for those patients with a diagnosis of high-risk MDS or TP53-mutant AML.
References
Competing Interests
Dr. Guirguis and Dr. Lane indicated no relevant conflicts of interest.