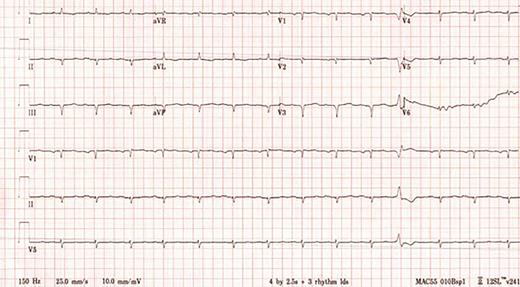
A 64-year-old woman presented in June 2019 to the emergency department (ED) for evaluation. She reported a mechanical fall resulting in a distal radius fracture and a distal ulnar fracture, and subsequently, acute left carpal tunnel syndrome and acute Guyon canal syndrome. A head computed tomography (CT) scan without contrast showed no acute intracranial abnormality. The patient underwent release of the left carpal tunnel and Guyon’s canal in the ED, with plans to return later for open reduction and internal fixation of the left distal radius fracture. Four days after her initial presentation, she returned to the ED, reporting bilateral headache and new visual loss. Electrocardiogram (ECG) was abnormal, showing Q waves in the inferior and anteroseptal leads and markedly low QRS voltage, especially in the limb leads (Figure 1). Troponin I was elevated at 0.13 ng/mL (normal, <0.03 ng/mL) as was N-terminal-pro hormone B-type natriuretic peptide (NT-proBNP) at 4,121 pg/mL (normal, <300 pg/mL). Cardiology was consulted for a preoperative assessment prior to the planned orthopedic surgery. A regadenoson myocardial perfusion scan was performed and showed no evidence of ischemia or infarction. The patient underwent surgery without complications and was discharged home on aspirin and a statin.
A 12-lead electrocardiogram showing sinus rhythm with Q waves in the anteroseptal and inferior leads as well as markedly low QRS voltage, particularly in the limb leads.
A 12-lead electrocardiogram showing sinus rhythm with Q waves in the anteroseptal and inferior leads as well as markedly low QRS voltage, particularly in the limb leads.
The patient was under the care of a cardiologist since 2017 when she reported lower extremity edema and dyspnea on exertion. More recently, these symptoms had progressed. Bilateral pleural effusions and persistent small pericardial effusion with normal left ventricular ejection fraction (LVEF) were identified by transthoracic echocardiogram (TTE) in February 2019. No unifying diagnosis could explain these findings. A second cardiologist evaluated her when she was in the ED, but still no unifying cardiac diagnosis was confirmed. In September 2019, the patient had progressive dyspnea and underwent a left heart catherization that showed angiographically normal epicardial coronary arteries with an elevated left ventricular end-diastolic pressure (LVEDP) of 20 mmHg, confirming heart failure with preserved ejection fraction. Her heart failure symptoms continued to worsen, requiring hospitalization for further management. A third cardiologist recommended evaluation for cardiac amyloidosis. Repeat TTE confirmed a normal LVEF, mild to moderate concentric hypertrophy, left atrial enlargement, and pericardial effusion in addition to apical sparing in strain imaging (Figure 2). Given the concern for cardiac amyloidosis, an endomyocardial biopsy was performed, confirming amyloid deposits and immunofluorescence that demonstrated the presence of λ light-chain deposition. Mass spectrometry completed in December 2019 confirmed the diagnosis as amyloid light-chain (AL) amyloidosis. Additional laboratory data can be found in Tables 1 and 2.
Two-dimensional transthoracic echocardiogram demonstrating the following: (A) Measuring the interventricular septal (IVS) and posterior wall (PW) thickness, both of which were determined to meet criteria for left ventricular hypertrophy; (B) Parasternal long axis view demonstrating the left ventricle (LV), left atrium (LA), and a small to moderate, posterior pericardial effusion (Peff); (C) strain imaging showing apical sparing, which has been associated with cardiac amyloidosis.
Two-dimensional transthoracic echocardiogram demonstrating the following: (A) Measuring the interventricular septal (IVS) and posterior wall (PW) thickness, both of which were determined to meet criteria for left ventricular hypertrophy; (B) Parasternal long axis view demonstrating the left ventricle (LV), left atrium (LA), and a small to moderate, posterior pericardial effusion (Peff); (C) strain imaging showing apical sparing, which has been associated with cardiac amyloidosis.
Troponin I and N-terminal-pro hormone B-type natriuretic peptide (NT-proBNP) Values Since the Patient’s Initial Presentation in June 2019
| Biomarker . | 6/28/19 . | 6/29/19 . | 9/15/19 . | 11/13/19 . | 11/14/19 . | 11/23/19 . | 12/27/19 . |
|---|---|---|---|---|---|---|---|
| Troponin I (ng/mL) | 0.13 | 0.17 | <0.3 | 0.19 | 0.29 | 0.55 | N/A |
| NT-proBNP (pg/mL) | N/A | 4,121 | N/A | 5,428 | N/A | N/A | 16,144 |
| Biomarker . | 6/28/19 . | 6/29/19 . | 9/15/19 . | 11/13/19 . | 11/14/19 . | 11/23/19 . | 12/27/19 . |
|---|---|---|---|---|---|---|---|
| Troponin I (ng/mL) | 0.13 | 0.17 | <0.3 | 0.19 | 0.29 | 0.55 | N/A |
| NT-proBNP (pg/mL) | N/A | 4,121 | N/A | 5,428 | N/A | N/A | 16,144 |
Basic Biochemistry Data for the Evaluation of Amyloidosis
| 11/4/2019 . | Result . | Reference . |
|---|---|---|
| Serum-free k light chain | 2.84 mg/dL | 0.33-1.94 mg/dL |
| Serum-free l light chain | 28.06 mg/dL | 0.57-2.63 mg/dL |
| Serum-free k/l light chain ratio | 0.10 | |
| Serum immunofixation | IgA l monoclonal protein detected | No monoclonal protein |
| 11/4/2019 . | Result . | Reference . |
|---|---|---|
| Serum-free k light chain | 2.84 mg/dL | 0.33-1.94 mg/dL |
| Serum-free l light chain | 28.06 mg/dL | 0.57-2.63 mg/dL |
| Serum-free k/l light chain ratio | 0.10 | |
| Serum immunofixation | IgA l monoclonal protein detected | No monoclonal protein |
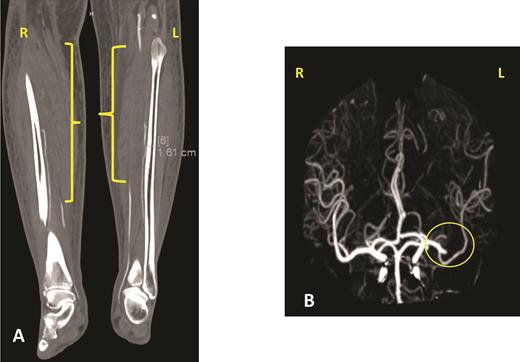
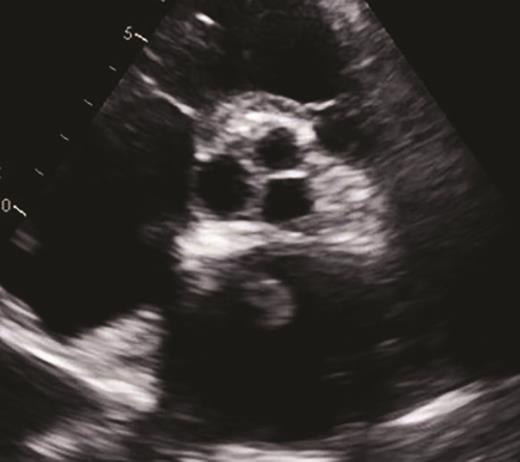
The patient’s hospital course in December 2019 was complicated by acute on chronic diastolic heart failure with preserved ejection fraction, new splenic infarct, acute lower extremity limb ischemia requiring revascularization, acute ischemic stroke involving the proximal inferior left M2 branch requiring mechanical thrombectomy, and a new lower extremity deep venous thrombus, all within a two-week period (Figure 3). After recovering from this hospitalization, the patient was started on treatment for AL amyloidosis with cyclophosphamide, bortezomib, and dexamethasone (CyBorD) in January 2020. Unfortunately, after one cycle of CyBorD, the patient developed gastrointestinal hemorrhage and recurrent stroke. A new left atrial thrombus was seen on TTE, despite ongoing anticoagulation with apixaban (Figure 4). The patient and family opted for comfort care at this time and the patient died shortly thereafter.
Panel A shows one of the views of a computed tomography angiogram of the lower extremities. The yellow brackets indicate areas of stenosis or occlusion in both lower extremities. The patient was found to have an occluded right popliteal artery, right anterior and posterior tibial artery, right tibioperoneal trunk, and right and left peroneal artery. She had different degrees of occlusion in those vessels. Panel B shows a cerebral angiogram, with the yellow circle indicating the occlusion of the proximal inferior left M2 branch.
Panel A shows one of the views of a computed tomography angiogram of the lower extremities. The yellow brackets indicate areas of stenosis or occlusion in both lower extremities. The patient was found to have an occluded right popliteal artery, right anterior and posterior tibial artery, right tibioperoneal trunk, and right and left peroneal artery. She had different degrees of occlusion in those vessels. Panel B shows a cerebral angiogram, with the yellow circle indicating the occlusion of the proximal inferior left M2 branch.
Short-axis view of a two-dimensional transthoracic echocardiogram demonstrating a new 1.5 × 1.0 cm echodensity (yellow circle) in the left atrium. This is compelling evidence by echocardiography indicating a thrombus.
Short-axis view of a two-dimensional transthoracic echocardiogram demonstrating a new 1.5 × 1.0 cm echodensity (yellow circle) in the left atrium. This is compelling evidence by echocardiography indicating a thrombus.
This case is a dramatic illustration of the challenges clinicians face in diagnosing cardiac amyloidosis. Many initial symptoms are nonspecific and may overlap with other causes of heart failure, commonly leading to delay in diagnosis. Abnormal cardiac biomarkers (troponin I and NT-proBNP) with low QRS voltage in the ECG should be red flags in patients with persistent heart failure symptoms (e.g., chronic dyspnea on exertion, pleural effusions, edema). Abnormal imaging, abnormal cardiac biomarkers, and a markedly abnormal ECG should all alert the clinician to this infiltrative condition. Early diagnosis can allow for initiation of disease-modifying therapy in what is otherwise a fatal illness.
While many hematologists are familiar with the heart failure, renal failure, and neuropathic complications of amyloidosis, this case highlights the vascular complications that can arise if diagnosis is delayed. A recent study showed a high cancellation rate of direct current cardioversion in patients with transthyretin cardiac amyloidosis due to a high incidence of intracardiac thrombus even among those who received adequate anticoagulation (31%).1 A similar pathogenesis is expected in patients with AL cardiac amyloidosis. It has also been shown that patients with plasma cell disorders have a higher risk of both venous and arterial thrombosis — a phenomenon associated with worse survival.2,-4 Thus, a timely diagnosis and immediate treatment initiation should be the goal.
References
Competing Interests
Dr. Alvarez-Cardona and Dr. Lenihan indicated no relevant conflicts of interest.