A new year is an opportunity to reflect. More than six years ago in 2014, the phenomenon of clonal hematopoiesis (CH) took the hematology community by storm.1-3 Despite the catchy moniker of “CHIP” (CH of indeterminate potential), the earliest papers did identify some initial associations with CH. Beyond the profound cardiovascular risk of individuals with CH, the risk of a subsequent hematologic malignancy was found to be 0.5 to 1 percent per year.1 Interestingly, these secondary hematologic neoplasms were of both myeloid and lymphoid lineage. In a separate study, flow cytometric cell sorting indeed identified CH not just in the myeloid cells, but in the lymphocytes as well, confirming that CH can involve all the hematopoietic compartments.4
Based on these first studies, the finding of CH mutations in lymphoid neoplasms should not have been a surprise. However, most of the later research centered on the development of myeloid neoplasms (MNs) from CH. This focus was largely directed by the finding of recurrent mutations in the “usual suspect” genes in both CH and MNs, especially epigenetic and splicing mutations.1-3,5 However, lymphoid malignancies, in particular lymphomas of follicular helper T-cell origin (TCL-TFHs), and less commonly other peripheral T-cell lymphomas, also have a notable association with some of these usual suspects. Specifically, TET2 and DNMT3A alterations are found in 76 percent and 33 percent of cases, respectively, of angioimmunoblastic T-cell lymphoma (AITL), the most common subtype of TCL-TFH.6 The initial finding in 2012 of these mutations in not only the neoplastic T cells but also in the B cells and myeloid cells, actually pre-dated the characterization of CH and was consequently phenomenological (and arguably underappreciated) at the time.7 An additional case was later reported of a patient with CH mutations in both their AITL and acute myeloid leukemia (AML), with AITL-specific acquisition of a RHOA mutation and an AML-specific NPM1 mutation.8
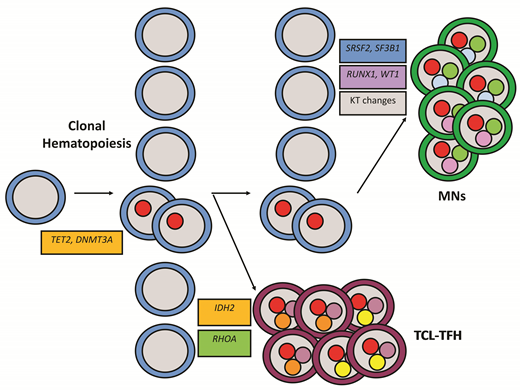
This past year, Dr. Natasha Lewis and colleagues successfully solidified our understanding of the clonal relationship between AITL and MNs.9 In 22 patients diagnosed with both a TCL-TFH and bone marrow (BM) CH, next-generation sequencing was performed on BM and tissue samples, with select cases also undergoing droplet digital polymerase chain reaction as well as flow cytometry sorting of BM and peripheral blood subpopulations. Within this cohort, identical mutations were detected in the neoplastic T cells and were detected or inferred to occur separately in the myeloid cells in 15 (68%) of 22 cases. TET2 was involved in 93 percent, and DNMT3A in 67 percent of the 15 cases. The AITLs contained additional clonally divergent mutations in other recurrent AITL genes, including RHOA and IDH2 among other less frequent genes (Figure). Similarly, the myeloid cells, both in cases with and without a defined MN, contained distinct divergent evolution with mutations in spliceosome components (SRSF2, SF3B1) and transcription factors (RUNX1, WT1), among other mutations and cytogenetic aberrations (Figure). Remarkably, four (18%) of 22 patients developed subsequent MNs, which also shared the genetic abnormalities of those patients’ previous T-cell lymphomas. In all four cases, the MN developed one to four years after initiation of AITL-directed chemotherapy and are technically considered therapy-related MNs (t-MNs).
Clonal evolution of lymphomas of follicular helper T-cell origin and myeloid neoplasms from high-risk clonal hematopoiesis due to mutations in TET2 and DNMT3A. Abbreviations: KT, karyotypic; MNs, myeloid neoplasms; TCL-TFH, lymphomas of follicular helper T-cell origin.
Clonal evolution of lymphomas of follicular helper T-cell origin and myeloid neoplasms from high-risk clonal hematopoiesis due to mutations in TET2 and DNMT3A. Abbreviations: KT, karyotypic; MNs, myeloid neoplasms; TCL-TFH, lymphomas of follicular helper T-cell origin.
The role of CH in t-MNs has been studied previously. In general, cancer survivors have a fourfold increased prevalence of CH, and CH is associated with increased risk of subsequent t-MNs.10-12 In many cases, the t-MNs are documented to be clonally related to the CH that was present prior to chemotherapy targeting their primary cancer. However, the CH mutations in these cases were NOT present in the primary tumor, even in those cases where the primary cancer was lymphoma.11 An additional scientific abstract demonstrated no clonal link between lymphoplasmacytic lymphoma cell populations and presumed myeloid elements harboring CHIP-defining mutations.13 Therefore, the clonal relatedness of MNs and TCL-TFHs seems to be unique among other subtypes of lymphoma.
Due to their shared genetics, one might reasonably hypothesize that TCL-TFHs would co-occur with MNs at an increased frequency. One epidemiologic study analyzed the Surveillance, Epidemiology, and End Results (SEER) database and demonstrated a higher rate of AML in patients previously diagnosed with AITL.14 Other rare case reports and series have described the development of MNs both before or after treatment for TCL-TFHs.7,8,15-18 Interestingly, all the cases of MNs in the study by Dr. Lewis and colleagues occurred after treatment of the AITL.9 Other studies have confirmed the potential expansion of CH clones in adverse BM environments after therapy, including auto- and allotransplantation.11,12,19
This study raised some important questions about the role of CH in TCL-TFHs. There was skewing of the normal patterns of CH in TCL-TFHs with higher rates of TET2 than DNMT3A mutations and low rates/absence of other recurrent CH genes such as ASXL1 and spliceosome components. Additionally, the associated myeloid compartments, including bona fide MNs, showed high co-occurrence of transcription factors, spliceosome components, and cytogenetic changes, but few signal transduction pathway mutations common in many MNs, especially more aggressive MNs. Finally, while this and other studies have identified the higher than expected rate of MNs in AITL, they do not address whether there is a higher rate of TCL-TFHs in CH. These are challenging questions to answer given the low prevalence of TCL-TFHs, the low rates of t-MNs, and the low prevalence of mutations other than those in TET2 and DNMT3A.
As we move forward into 2021, we should embrace the clonal similarities between diagnostically disparate entities and view TCL-TFHs as a category of lymphoid diseases that shares common hematopoietic origins with MNs. This concept of clonal relatedness with distinct pathologic manifestations is not new. Indeed, within the myeloid realm, the clonal relationship of distinct presentations is well-documented.20,21 Even within the lymphoid realm, clonal evolution is common in the transformation from small B-cell neoplasms to diffuse large B-cell lymphoma and some cases of classic Hodgkin lymphoma.22 However, Dr. Lewis and colleagues have documented a clear clonal relationship beginning with a foundation of CH in progenitor cells and leading to distinct neoplasms that cross traditional lineage barriers. This study encourages all of us to look more holistically at hematologic neoplasms. Perhaps, as in so many other walks of life, groups that on the surface are highly disparate, in fact, share more in common than what sets them apart.
References
Competing Interests
Dr. Evans and Dr. Kim indicated no relevant conflicts of interest.