Study Title: The Effect of Exercise on Resting Biomarkers in Subjects With Sickle Cell Trait (SCT)
Clinicaltrials.Gov Identifier: NCT04273022
Sponsor: St. Louis University with collaboration of the American Society for Clinical Laboratory Science
Participating Centers: St. Louis University
Accrual Goal: 20 participants (15 with SCT, 5 without SCT)
Study Design: Interventional nonrandomized single-group assignment clinical trial
Summary: This nonrandomized clinical trial is enrolling healthy volunteers of all genders between ages 18 and 70 years, who have SCT (Hgb AS) and a control group of healthy subjects without SCT (Hgb AA). The study will evaluate the impact of a single burst of standardized moderate intensity exercise on 15 biomarkers that represent five physiological pathways (Table) and compare those values between those participants with and without SCT. Exclusion criteria include weight less than 110 lbs.; pregnancy; having a hemoglobinopathy other than SCT determined by hemoglobin subtype quantification using electrophoresis; presence of a self-reported condition known to cause blood hypercoagulation activation, monocyte destruction hemolysis, chronic inflammation, or renal disease; and/or any condition that places the individual at increased risk during exercise.
| Biomarkers of Interest . | Physiological Pathway . |
|---|---|
| Reticulocyte | H |
| Red blood cell morphology | H, M, T, I, R |
| Haptoglobin | H |
| Serum potassium | H |
| Serum creatinine kinase | M |
| Serum myoglobin | M |
| Urine myoglobin | M |
| D-dimer | T |
| Fibrin monomer | T |
| Anti-thrombin III | T |
| C-reactive protein | I |
| Erythrocyte sedimentation rate | I |
| 11-dehydrothrombaxaneB2 (11-DTXB2) level | I |
| Complete urinalysis | H, R |
| Microalbumin | R |
| Biomarkers of Interest . | Physiological Pathway . |
|---|---|
| Reticulocyte | H |
| Red blood cell morphology | H, M, T, I, R |
| Haptoglobin | H |
| Serum potassium | H |
| Serum creatinine kinase | M |
| Serum myoglobin | M |
| Urine myoglobin | M |
| D-dimer | T |
| Fibrin monomer | T |
| Anti-thrombin III | T |
| C-reactive protein | I |
| Erythrocyte sedimentation rate | I |
| 11-dehydrothrombaxaneB2 (11-DTXB2) level | I |
| Complete urinalysis | H, R |
| Microalbumin | R |
Abbreviations: H, hemolysis; I, inflammation; M, myolysis; R, renal function; T, thrombosis.
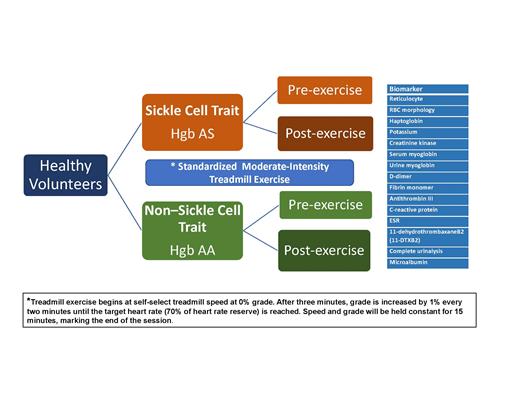
After consent, screening, and enrollment, each participant will undergo a single bout of moderate exercise that is standardized using a treadmill (Figure 1). Blood and urine samples will be obtained at three time points: before exercise, immediately after completing the exercise activity, and 24 hours after exercise.
Treatment schema. ESR, erythrocyte sedimentation rate; RBC, red blood cell.
Treatment schema. ESR, erythrocyte sedimentation rate; RBC, red blood cell.
The primary endpoints of this study are the differences in response to moderate-intensity exercise between SCT and non-SCT individuals in the biomarkers listed in the Table.
Rationale: The physiological pathways of interest (hemolysis, inflammation, thrombosis, myolysis, and renal function) have been implicated in adverse events among athletes and military personnel with SCT following repeated bouts of prolonged intense exertion.1 There is a significant knowledge gap regarding the expected dose-dependent physiological biomarker response to physical exertion in SCT, thereby making it impossible to provide objective guidance on what should be considered potentially dangerous physical exertion. Therefore, detecting changes in biomarkers after standardized activity in SCT compared to non-SCT controls will provide insight into the degree to which these pathways are differentially activated in SCT before and after exercise and the potential clinical and subclinical implications of these changes.
When compared to controls without SCT, the finding of abnormal physiological biomarkers in SCT may suggest that there is chronic activation of certain pathways. Post-exercise changes in these biomarkers may suggest further activation in response to exertion. The investigators will evaluate biomarker levels at 24 hours following exercise to determine if there is continued activation or recovery of involved pathways as levels are expected to return to pre-exercise levels after 24 hours.
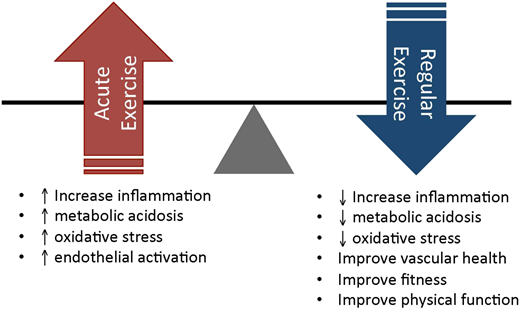
Comment: While there are concerns that exercise-induced aberrant activation of pathophysiological pathways may predispose to individuals with SCT to adverse outcomes such as thrombosis, acute kidney injury, heightened inflammation, hemolysis, and myolysis, the degree of risk is not clearly elucidated. Numerous epidemiological studies have shown that cardiopulmonary fitness is a primary predictor of all-cause mortality among all individuals — those with or without SCT and also those with or without chronic medical conditions.2 It is important to not throw the baby (the benefits of regular exercise to promote cardiovascular health) out along with the bathwater (the potential negative physiologic consequences of acute intense exercise that may trigger inflammation, metabolic acidosis, oxidative stress, and endothelial activation), as is elegantly depicted by Dr. Robert I. Liem in Figure 2. This study is therefore timely as it will help elucidate the differences in physiologic response to exertion between SCT and non-SCT individuals, providing much needed preliminary data to guide further studies on the dose of exertion that may or may not be safe for individuals with SCT.
The balance of short-term potential risks of acute exercise vs long-term benefits of regular exercise in individuals with SCT and SCA. From Liem RI. Hematology Am Soc Hematol Educ Program. 2018;2018:418-425.
The balance of short-term potential risks of acute exercise vs long-term benefits of regular exercise in individuals with SCT and SCA. From Liem RI. Hematology Am Soc Hematol Educ Program. 2018;2018:418-425.
Various strategies have been recommended to reduce the risk of adverse outcomes following overexertion in individuals with SCT. These may include graduation in training intensity escalation over time; liberal hydration during training; avoiding intense training after prolonged illness or rest; and widespread education about early signs of heat-related illness, exertional rhabdomyolysis, and exercise collapse associated with SCT. Promptly terminating training and implementing treatment if symptoms develop is recommended. None of these recommendations are based on high-quality evidence but rely on consensus statements and expert opinion. This study will provide additional evidence to ultimately support the development of evidence-based guidelines that provide safe training and exercise suggestions for individuals living with SCT who comprise nearly 8 percent of the black population in the United States.
References
Competing Interests
Dr. Osunkwo indicated no relevant conflicts of interest.